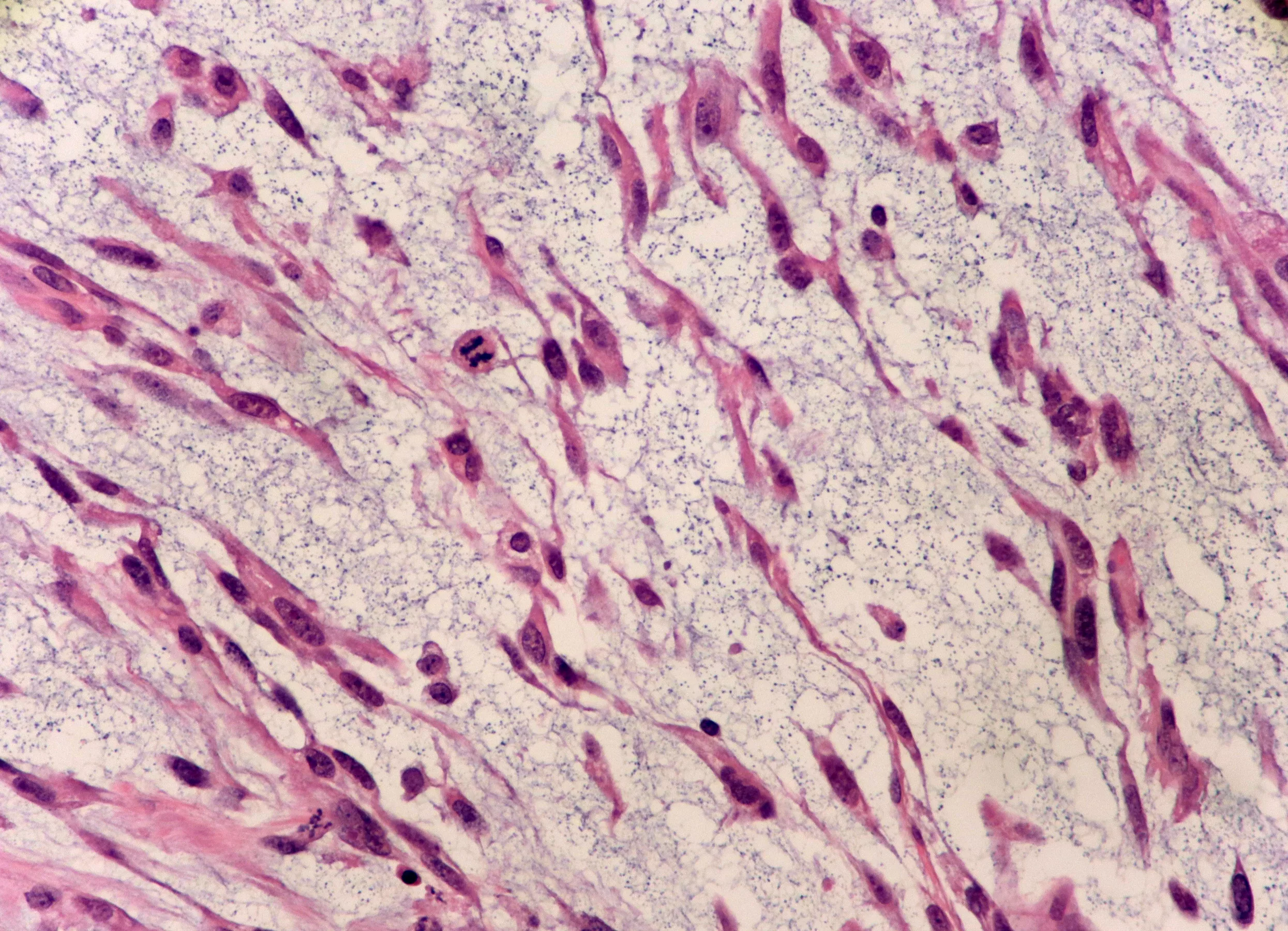
Myxofibrosarcoma (MFS) is a rare type of cancer that forms in the connective (fibrous) tissues. MFS is a type of myxoid soft tissue sarcomas (STS). STSs are heterogeneous mesenchymal neoplasms characterized by myxoid stroma (a gelatinous, mucin-rich extracellular matrix that supports tumor cells and influences tumor behavior). According to the 2020 World Health Organization Classification of Soft Tissue Tumors, it belongs to the myofibroblastic or fibroblastic tumor group. MFS can occur at any age. Though it has a slight male predominance, its peak incidence is in the sixth to eighth decades of life. As it is a rare condition, its estimated incidence is less than 0.1 per 100,000 individuals annually.1Nishio, J. and S. Nakayama, Biology and management of high-grade myxofibrosarcoma: state of the art and future perspectives. Diagnostics, 2023. 13(19): p. 3022.
MFS arises in the extremities of elderly patients. It represents a slow-growing or painless mass that usually occurs under the skin of the legs and arms. It often exhibits an infiltrative growth pattern, which increases the risk of local recurrence even after surgical removal. In some cases, multiple tumor nodules may form within the affected region, and while MFS primarily spreads locally, distant metastases—most commonly to the lungs—can occur. Surgery remains the primary treatment approach, with wide excision being crucial to reduce the high recurrence rates, which range from 10% to 61%. Due to its aggressive local behavior, careful long-term monitoring is necessary to manage potential recurrences.2Nishio, J. and S. Nakayama, Biology and management of high-grade myxofibrosarcoma: state of the art and future perspectives. Diagnostics, 2023. 13(19): p. 3022.
Causes of Myxofibrosarcoma
The exact cause of MFS is unknown. Like all other types of cancer, it can develop with specific mutations or modifications in the cellular material. A change happens in the DNA of the connective tissue cells. This change commands your cells to multiply irregularly and grow abnormally without dying, causing them to accumulate and form a tumor mass.
Symptoms / Clinical Presentation of MFS
The symptoms of MFS depend on the location and origin of the tumor. Most MFS tumors develop in the subcutaneous tissues, while others infiltrate the underlying fascia or skeletal muscle. Early on, symptoms may be absent or minimal. Over time, a painless, slow-growing lump may appear under the skin. In some cases, patients experience discomfort, localized swelling, or systemic inflammatory responses.3Sambri, A., et al., Systemic inflammation is associated with oncological outcome in patients with high-grade myxofibrosarcoma of the extremities: a retrospective analysis. Oncology research and treatment, 2020. 43(10): p. 531-538.
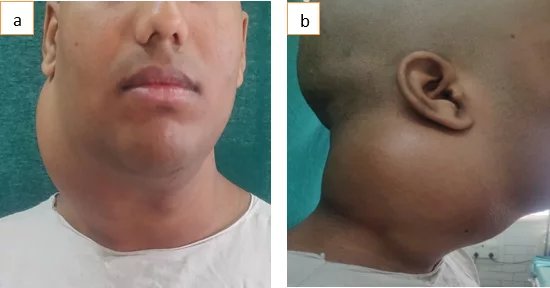
Stages of MFS
The staging system of STS relies on four pieces of information. These key pieces include:
- The extent of tumor (T): What is the size of the cancer?
- Spread to neighboring lymph nodes (N): Has the sarcoma spread to nearby lymph nodes?
- Metastasis (M): Has cancer spread to distant organs?
- Grade (G): how much does the sarcoma resemble normal cells?
The French Fédération Nationale des Centres de Lutte Contre le Cancer (FNCLCC) system is commonly used to classify MFS into three histological grades based on:
- Differentiation – How closely the tumor cells resemble normal connective tissue cells.
- Mitotic count – The number of dividing cells, indicating how quickly the tumor is growing.
- Tumor necrosis – The presence of dead tumor cells, suggesting rapid growth and poor oxygen supply.
The Three Grades of MFS are the following:
Low-Grade MFS:
They consist of spindle cells with atypical hyperchromatic nuclei in the variably myxoid matrix. Tumor necrosis is absent in low-grade MFS. Mitotic figures are rare, and pseudo-blasts contain cytoplasmic mucin.4Anderson, W.J. and L.A. Doyle, Updates from the 2020 World Health Organization classification of soft tissue and bone tumours. Histopathology, 2021. 78(5): p. 644-657.
Intermediate-Grade MFS:
They are more cellular and pleomorphic. They contain minute solid areas with flank pleomorphism. These lack tumor necrosis or it is minimal. Mitotic figures are more frequent than in low-grade MFS but remain below high-grade thresholds.
High-Grade MFS:
This type of MFS contains marked nuclear pleomorphism, including spindle cells and pleomorphic giant cells. Tumor necrosis and atypical mitoses are variably present. Mitotic figures exceed 10 mitoses per 10 high-power fields, indicating aggressive behavior.5Nishio, J. and S. Nakayama, Biology and management of high-grade myxofibrosarcoma: state of the art and future perspectives. Diagnostics, 2023. 13(19): p. 3022.
Risk Factors
Some factors can increase the risk of MFS. The risk factors include:
- Environmental Factors
Some specific environmental factors like chemicals (herbicides, arsenic) can increase the risk of developing MFS. - Genetic Factors
Mutations of certain genes can raise the risk of STS. - Treatment-Related Factors
A history of radiation therapy can increase the risk of developing MFS in individuals. - Age
Older age is also a risk factor for developing MFS. - Family History
People with a family history of STS or any other cancer are more inclined to develop MFS.
Diagnosis of MFS
Diagnosing MFS is a challenging task. However, it involves a combination of physical examination, imaging tests, and biopsy.
Physical Assessment:
The healthcare provider will ask for the symptoms and your family history. He will thoroughly examine the affected area and look for lumps.
Imaging Tests:
Imaging plays a crucial role in diagnosing MFS, determining tumor extent, and planning treatment. Common imaging modalities include ultrasonography (US), magnetic resonance imaging (MRI), computed tomography (CT), and positron emission tomography (PET).
Ultrasonography (US)
In the US, MFS display a predominantly hypoechoic heterogeneous mass, tail-like extensions along the fascial layer, or ill-defined echogenic alterations in the neighboring subcutaneous fat.6Morag, Y. and D.R. Lucas, Ultrasound of myxofibrosarcoma. Skeletal radiology, 2022: p. 1-10. It is the first-line modality for assessing visible and palpable superficial soft tissue masses. Moreover, it helps in directing the percutaneous biopsies.
Magnetic Resonance Imaging (MRI)
MRI is the most sensitive imaging tool for assessing MFS. It helps distinguish the tumor from surrounding tissues and is essential for surgical planning. A characteristic “tail sign”—an infiltrative extension along the fascial plane—is often seen, indicating a high risk of local recurrence. High-grade MFS may also exhibit necrosis and hemorrhage on MRI.7Nishio, J. and S. Nakayama, Biology and management of high-grade myxofibrosarcoma: state of the art and future perspectives. Diagnostics, 2023. 13(19): p. 3022.
CT & PET Scans
CT and PET scans help assess deeper tumors and detect distant metastases. PET scans detect metabolic activity using fluorodeoxyglucose (FDG) uptake. Low-grade MFS typically exhibits lower FDG uptake, whereas high-grade MFS demonstrates increased uptake due to its aggressive nature.8Macpherson, R.E., et al., Retrospective Audit of 957 Consecutive 18F-FDG PET–CT Scans Compared to CT and MRI in 493 Patients with Different Histological Subtypes of Bone and Soft Tissue Sarcoma. Clinical Sarcoma Research, 2018. 8: p. 1-12. PET/CT also helps guide biopsy by identifying the most metabolically active tumor regions.9Hain, S., et al., Can FDG PET Be Used to Successfully Direct Preoperative Biopsy of Soft Tissue Tumors? Nuclear Medicine Communications, 2003. 24(11): p. 1139-1143.
Chest X-Ray
Chest X-ray will help in finding whether the cancer has spread to your lungs or not. It mostly happens when the cancer metastasizes.
Biopsy:
A core needle biopsy is the preferred method for diagnosing MFS, as it provides an adequate tissue sample for histopathological examination. A fine needle aspiration (FNA) biopsy is generally insufficient for sarcomas. The biopsy sample is examined for cellular characteristics, mitotic activity, and necrosis to determine tumor grade.
The findings of all diagnostic modalities help in finding the stage of the tumors and executing an appropriate treatment plan.
Treatment & Management of MFS
The most common treatment for MFS is surgery. However, radiation and chemotherapy are also suitable options for treating and managing the condition.
Surgery:
Surgery is the standard treatment for localized MFS, aiming for wide excision with negative margins (no cancer cells at the edges). The surgical procedure selection is according to the tumor’s size, stage, and location. Deep intramuscular masses require a composite reconstruction, such as a skin graft or muscle flap. Surgery can be challenging due to infiltrative growth. MFS can recur after surgical resection of the tumor. The local recurrence rates are between 50% to 60%.10Barretina, J., et al., Subtype-specific genomic alterations define new targets for soft-tissue sarcoma therapy. Nature genetics, 2010. 42(8): p. 715-721. Margin assessment is critical to predict the risk of local recurrence. However, there is a lack of data defining the adequate margin for resection for MFS.11Vanni, S., et al., Myxofibrosarcoma landscape: Diagnostic pitfalls, clinical management and future perspectives. Therapeutic Advances in Medical Oncology, 2022. 14: p. 17588359221093973.
Radiation Therapy (RT):
The beneficial effect of RT on local tumors for treating MFS and clearing it out is still controversial. The healthcare provider uses adjuvant or neoadjuvant treatment strategies to treat local tumors. The adjuvant treatment of RT with surgery has a lower risk of local recurrence. The drawbacks linked with RT include:
- Pain
- Poor wound healing
- Fibrosis
- Risk of secondary neoplasm
- Edema
Chemotherapy:
Chemotherapy is not standard for localized MFS but may be considered for high-grade or metastatic cases. Common chemotherapy regimens include:
Gemcitabine-Based Therapy
The providers use gemcitabine (intravenous 1200 mg/m2 day one to eight every 21 days) as monotherapy in combination with dacarbazine and docetaxel (900 mg/ m2 intravenous) in STS patients. However, the exact role of gemcitabine is still unclear. It is usually an effective option for metastatic MFS patients.12Maki, R.G., et al., Randomized phase II study of gemcitabine and docetaxel compared with gemcitabine alone in patients with metastatic soft tissue sarcomas: results of sarcoma alliance for research through collaboration study 002. Journal of Clinical Oncology, 2007. 25(19): p. 2755-2763.
Anthracycline-Based Therapy
Like all other STS subtypes, anthracycline (105mg/sqm, three days, every three weeks) with or without ifosfamide (9000 mg/sqm, three days, every three weeks) is the first-line treatment for advanced MFS. It shows a significant improvement in MFS patients.13Colia, V., et al., Activity of anthracycline-and ifosfamide-based chemotherapy in a series of patients affected by advanced myxofibrosarcoma. Clinical Sarcoma Research, 2017. 7: p. 1-7.
Trabectedin
Administration of trabectedin (1.5mg/m2 every 24 hrs for three weeks) in patients with advanced STS is safe and effective. United States Food and Drug Administration (FDA) approved it for unresectable and metastatic liposarcoma patients.14akamura, T. and A. Sudo, The role of trabectedin in soft tissue sarcoma. Frontiers in Pharmacology, 2022. 13: p. 777872.
Other Drugs
Some other drugs, like pazopanib and eribulin, are also efficient in treating MFS. Pazopanib posses anti-tumorigenic and angiogenic properties. It has been approved as a second-line treatment for patients with STS in several countries. Eribulin is licensed for use in patients with metastatic liposarcoma and all other types of STS.15Nishio, J. and S. Nakayama, Biology and management of high-grade myxofibrosarcoma: state of the art and future perspectives. Diagnostics, 2023. 13(19): p. 3022.
Alternative Treatment Strategy:
Targeted therapies can target specific growth pathways involved in cancer development and inhibit the development of cancer cells. The healthcare provider uses immunotherapy to treat MFS. Immunotherapy uses the body’s immune system to fight the cancer cells.
Cryotherapy and High-intensity Focused Ultrasound (HIFU) are minimally invasive techniques that target tumors or tissues within the body. More studies are required to evaluate the effectiveness of HIFU and cryotherapy as a promising treatment for MFS.16Nishio, J. and S. Nakayama, Biology and management of high-grade myxofibrosarcoma: state of the art and future perspectives. Diagnostics, 2023. 13(19): p. 3022.
Prognosis of MFS
The prognosis of MFS depends upon the tumor’s grade, size, and location. MFS is likely to recur even after treatment. The high-grade tumors are more likely to return than low- and intermediate-grade MFS. Previous studies identified that high mitotic rate and high tumor grade are the main prognostic factors for worse survival in MFS.17Abdou, M., et al., Myxofibrosarcoma: Outcomes, Prognostic Factors, and Role of Neoadjuvant Radiation Therapy. Advances in Radiation Oncology, 2024. 9(6): p. 101485.
MFS versus Fibromyxoid Sarcoma (FMS)
MFS and FMS are two different types of STS. They differ in their demographic patterns, histopathology, and clinical presentation. The key differences between MFS and FMS are given below in the Table:
| MFS | FMS |
| A malignant soft tissue tumor.
It is a slow-growing, painless mass. MFS is seen in every age group but is more common in people aged 50 to 60. |
It is a low-grade tumor.
Generally less aggressive than MFS. Typically present in younger adults. |
| The histological features include curvilinear vessels and pleomorphic cells. | The histological features include a whorled pattern and bland spindle cells. |
| The growth patterns of MFS are infiltrative and multinodular. | The growth patterns of FMS are linear or whorled. |
| Common locations of MFS are subcutaneous or superficial. | Common locations of FMS are deep soft tissues. |
| This kind of tumor exhibits varying necrosis and hypocellularity, especially in high-grade cases. | FMS lacks significant necrosis and nuclear pleomorphism. |
| It possesses high rates of local recurrence. Recurrence often manifests in high-grade tumor cases. However, the metastatic potential is less than that of other sarcomas. | FMS can metastasize in more than half of the cases.
Its malignancy potential is high. |
Final Remarks
MFS is a type of STS that often starts as a small, painless lump under the skin. Symptoms of MFS appear slowly with the growth of the lump. These symptoms include systemic inflammation, pain, and discomfort. The management of MFS is challenging. The healthcare providers treat and manage the ailment through surgery, chemotherapy, and radiation therapy. However, even after appropriate treatment, MFS is more likely to return. Novel therapeutic strategies can lead to a substantial improvement in the outcomes of patients with this devastating disorder. Follow-up appointments are a must to eliminate the disorder completely.
Refrences
- 1Nishio, J. and S. Nakayama, Biology and management of high-grade myxofibrosarcoma: state of the art and future perspectives. Diagnostics, 2023. 13(19): p. 3022.
- 2Nishio, J. and S. Nakayama, Biology and management of high-grade myxofibrosarcoma: state of the art and future perspectives. Diagnostics, 2023. 13(19): p. 3022.
- 3Sambri, A., et al., Systemic inflammation is associated with oncological outcome in patients with high-grade myxofibrosarcoma of the extremities: a retrospective analysis. Oncology research and treatment, 2020. 43(10): p. 531-538.
- 4Anderson, W.J. and L.A. Doyle, Updates from the 2020 World Health Organization classification of soft tissue and bone tumours. Histopathology, 2021. 78(5): p. 644-657.
- 5Nishio, J. and S. Nakayama, Biology and management of high-grade myxofibrosarcoma: state of the art and future perspectives. Diagnostics, 2023. 13(19): p. 3022.
- 6Morag, Y. and D.R. Lucas, Ultrasound of myxofibrosarcoma. Skeletal radiology, 2022: p. 1-10.
- 7Nishio, J. and S. Nakayama, Biology and management of high-grade myxofibrosarcoma: state of the art and future perspectives. Diagnostics, 2023. 13(19): p. 3022.
- 8Macpherson, R.E., et al., Retrospective Audit of 957 Consecutive 18F-FDG PET–CT Scans Compared to CT and MRI in 493 Patients with Different Histological Subtypes of Bone and Soft Tissue Sarcoma. Clinical Sarcoma Research, 2018. 8: p. 1-12.
- 9Hain, S., et al., Can FDG PET Be Used to Successfully Direct Preoperative Biopsy of Soft Tissue Tumors? Nuclear Medicine Communications, 2003. 24(11): p. 1139-1143.
- 10Barretina, J., et al., Subtype-specific genomic alterations define new targets for soft-tissue sarcoma therapy. Nature genetics, 2010. 42(8): p. 715-721.
- 11Vanni, S., et al., Myxofibrosarcoma landscape: Diagnostic pitfalls, clinical management and future perspectives. Therapeutic Advances in Medical Oncology, 2022. 14: p. 17588359221093973.
- 12Maki, R.G., et al., Randomized phase II study of gemcitabine and docetaxel compared with gemcitabine alone in patients with metastatic soft tissue sarcomas: results of sarcoma alliance for research through collaboration study 002. Journal of Clinical Oncology, 2007. 25(19): p. 2755-2763.
- 13Colia, V., et al., Activity of anthracycline-and ifosfamide-based chemotherapy in a series of patients affected by advanced myxofibrosarcoma. Clinical Sarcoma Research, 2017. 7: p. 1-7.
- 14akamura, T. and A. Sudo, The role of trabectedin in soft tissue sarcoma. Frontiers in Pharmacology, 2022. 13: p. 777872.
- 15Nishio, J. and S. Nakayama, Biology and management of high-grade myxofibrosarcoma: state of the art and future perspectives. Diagnostics, 2023. 13(19): p. 3022.
- 16Nishio, J. and S. Nakayama, Biology and management of high-grade myxofibrosarcoma: state of the art and future perspectives. Diagnostics, 2023. 13(19): p. 3022.
- 17Abdou, M., et al., Myxofibrosarcoma: Outcomes, Prognostic Factors, and Role of Neoadjuvant Radiation Therapy. Advances in Radiation Oncology, 2024. 9(6): p. 101485.