Erythroblastosis Fetalis, also known as hemolytic disease of the fetus and newborn (HDFN), is a largely preventable condition. It’s a serious and potentially life-threatening disease that occurs when there’s an “incompatibility” between the blood types of a mother and the fetus. In short, the mother’s body identifies the baby’s red blood cells (RBCs) as foreign and begins to destroy them.
Because of modern advances in prenatal care, and particularly, in Rh immunoprophylaxis, this condition has almost been eradicated in well-resourced healthcare settings. In other parts of the world, with poor access to antenatal screening and preventive treatment, erythroblastosis fetalis remains a threat to the health of newborns.
Pathophysiology of Erythroblastosis Fetalis
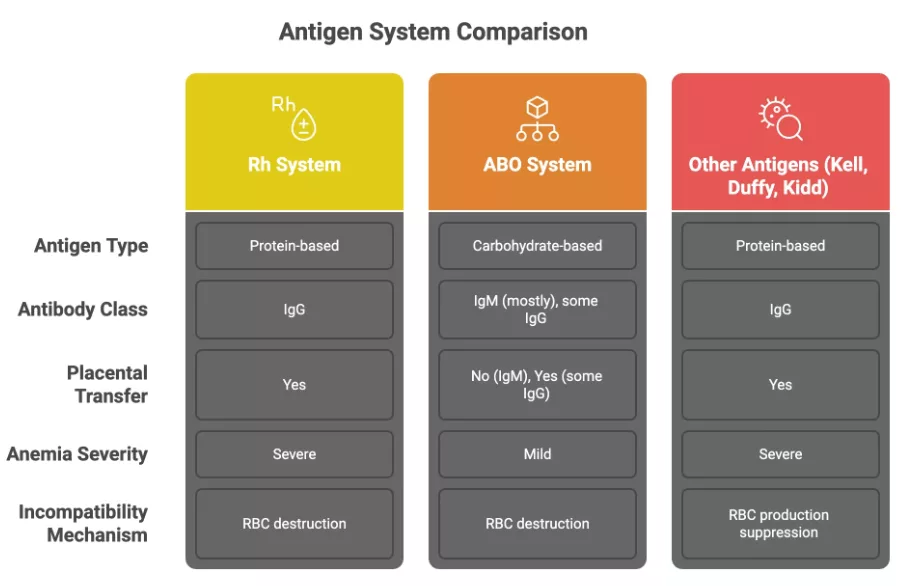
Most cases of erythroblastosis are because of Rh(D) and ABO incompatibilities, with the underlying mechanism being: immune-mediated hemolysis.
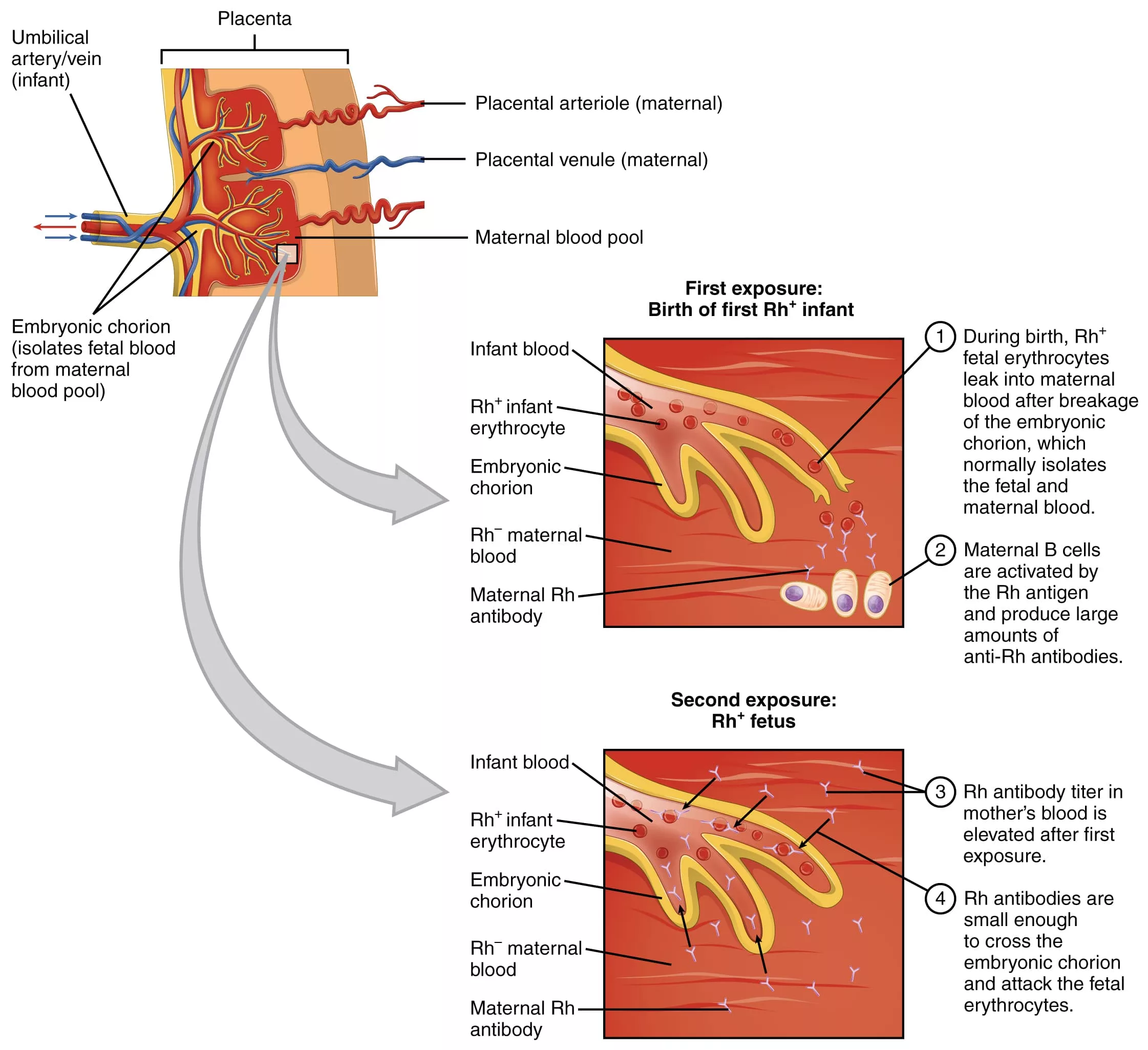
This hemolysis (breakdown of red blood cells) is due to incompatibility between maternal and fetal blood types. The problem begins when fetal RBCs carrying antigens inherited from the father enter the maternal bloodstream. This can happen during delivery, miscarriage, trauma, or invasive procedures like amniocentesis. Once this exposure occurs, the maternal immune system may recognize these antigens as foreign and produce antibodies against them.
Rh(D) Incompatibility:
The Rh factor refers to a group of proteins on the surface of RBCs, the most important of which is the D antigen. If it’s present, you’re Rh-positive. If not, you’re Rh-negative.
- If an Rh-negative mother is carrying an Rh-positive fetus, her immune system may recognize the D antigen as foreign. The first exposure typically doesn’t cause harm. The maternal immune system makes IgM antibodies, which are large and don’t cross the placenta.
- In later pregnancies, though, re-exposure causes a switch to IgG antibodies.1Moinuddin, I., Fletcher, C., & Millward, P. (2019). Prevalence and specificity of clinically significant red cell alloantibodies in pregnant women – a study from a tertiary care hospital in Southeast Michigan. Journal of blood medicine, 10, 283–289. https://doi.org/10.2147/JBM.S214118 These cross the placenta, bind to fetal red blood cells, and mark them for destruction. This leads to hemolysis and progressively worsening anemia in the fetus.
- To compensate, the fetal bone marrow speeds up RBC production, pushing out immature RBCs, erythroblasts, into the circulation. Hence the name: erythroblastosis fetalis.
- If hemolysis is severe, the fetal heart struggles to keep up with the oxygen demands. This can lead to high-output heart failure, fluid buildup in the organs and tissues (hydrops fetalis), and intrauterine death.
ABO Incompatibility:
ABO-related hemolytic disease works similarly but is usually milder. Mothers with type O blood naturally carry anti-A and anti-B antibodies that can sometimes cross the placenta and destroy fetal red cells with A or B blood. However, ABO antigens are less densely expressed on fetal red cells, making this form typically less severe than Rh-mediated HDFN.
Other Blood Group Antibodies:
And it’s not just Rh and ABO. Rare cases of HDFN can be triggered by antibodies to Kell, Duffy, or Kidd antigens. Some, like anti-Kell, are especially concerning because they inhibit red cell production at the bone marrow level, leading to more severe anemia.2Ohto, H., Denomme, G. A., Ito, S., Ishida, A., Nollet, K. E., & Yasuda, H. (2020). Three non-classical mechanisms for anemic disease of the fetus and newborn, based on maternal anti-Kell, anti-Ge3, anti-M, and anti-Jra cases. Transfusion and apheresis science : official journal of the World Apheresis Association : official journal of the European Society for Haemapheresis, 59(5), 102949. https://doi.org/10.1016/j.transci.2020.102949
Prevalence of Erythroblastosis Fetalis
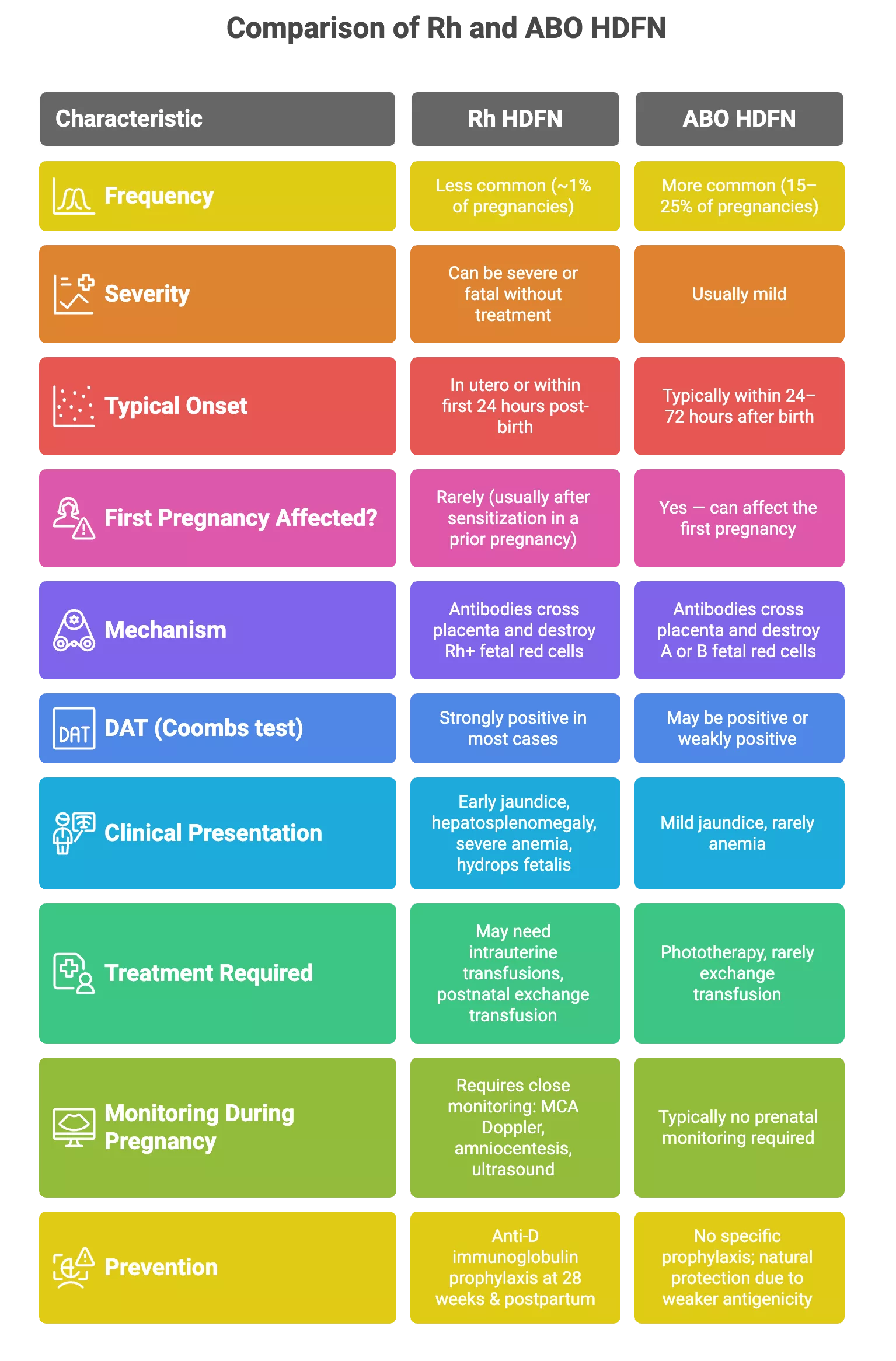
Erythroblastosis fetalis affects around 1–2% of pregnancies worldwide (translating to roughly 1,695 cases per 100,000 live births), but the numbers vary dramatically based on the type of incompatibility.3Gabbay, J. M., Agneta, E. M., Turkington, S., Bajaj, B. M., Sinha, B., & Geha, T. (2023). Rates of phototherapy among ABO-incompatible newborns with a negative direct antiglobulin test. Journal of perinatology : official journal of the California Perinatal Association, 43(11), 1357–1362. https://doi.org/10.1038/s41372-023-01650-3
- ABO incompatibility is common, showing up in 15–25% of pregnancies, but most remain mild and fewer than 1% evolve into clinically significant disease.
- Rh incompatibility, on the other hand, is rare overall, but the effects are often more severe.
The introduction of Rh immunoglobulin (RhoGAM) dramatically dropped Rh-induced HDFN from 99 to 44 cases per 100,000 live births in countries with routine screening, and mortality has been cut by nearly two-thirds.
But in regions with limited healthcare resources, across sub-Saharan Africa, South Asia, and parts of Latin America, Rh incompatibility remains a major concern. In Pakistan, for example, an estimated 270,000 Rh-incompatible pregnancies occur annually, still as many as 87% of affected women don’t receive prophylaxis.4Zipursky A, Bhutani VK. Impact of Rhesus disease on the global problem of bilirubin-induced neurologic dysfunction. Semin Fetal Neonatal Med. 2015 Feb;20(1):2-5. doi: 10.1016/j.siny.2014.12.001. Epub 2015 Jan 9. PMID: 25582277. This is a reflection of real-world inequities: limited healthcare infrastructure, gaps in antenatal screening, and poor access to Rh immunoglobulin continue to put newborns at risk.
Causes & Risk Factors of Erythroblastosis Fetalis
The root cause of erythroblastosis fetalis is that the mother’s immune system becomes sensitized to fetal red blood cell antigens and begins producing antibodies that target the fetus’s red cells for destruction. This can occur due to several risk factors:
- Rh-negative mother carrying an Rh-positive fetus (the most common cause of severe HDFN)
- Previous sensitizing events, including:
- Birth of a Rh-positive infant
- Miscarriage or abortion
- Invasive prenatal procedures (amniocentesis or chorionic villus sampling)
- Abdominal trauma during pregnancy
- Ethnicity: Rh-negative status is more common in Caucasians (15–16%) than Asians (<1%)5Myle, A. K., & Al-Khattabi, G. H. (2021). Hemolytic Disease of the Newborn: A Review of Current Trends and Prospects. Pediatric health, medicine and therapeutics, 12, 491–498. https://doi.org/10.2147/PHMT.S327032
- Inadequate or missed administration of anti-D immunoglobulin after a sensitizing event
- Multiparity, especially without Rh prophylaxis
Clinical Presentation of Erythroblastosis Fetalis
The signs and symptoms of Erythroblastosis fetalis depend on how early it’s detected and how severe the RBC breakdown is.
Before Birth (Prenatal Signs):
In the womb, the degree of hemolysis determines the severity of clinical signs:
- Mild cases may be asymptomatic or result in mild anemia.
- Moderate to severe cases lead to:
- Fetal anemia
- Hepatosplenomegaly from extramedullary hematopoiesis
- Ascites, pleural effusion, and subcutaneous edema (signs of hydrops fetalis)6Myle, A. K., & Al-Khattabi, G. H. (2021). Hemolytic Disease of the Newborn: A Review of Current Trends and Prospects. Pediatric health, medicine and therapeutics, 12, 491–498. https://doi.org/10.2147/PHMT.S327032
- Cardiac failure due to high-output physiology
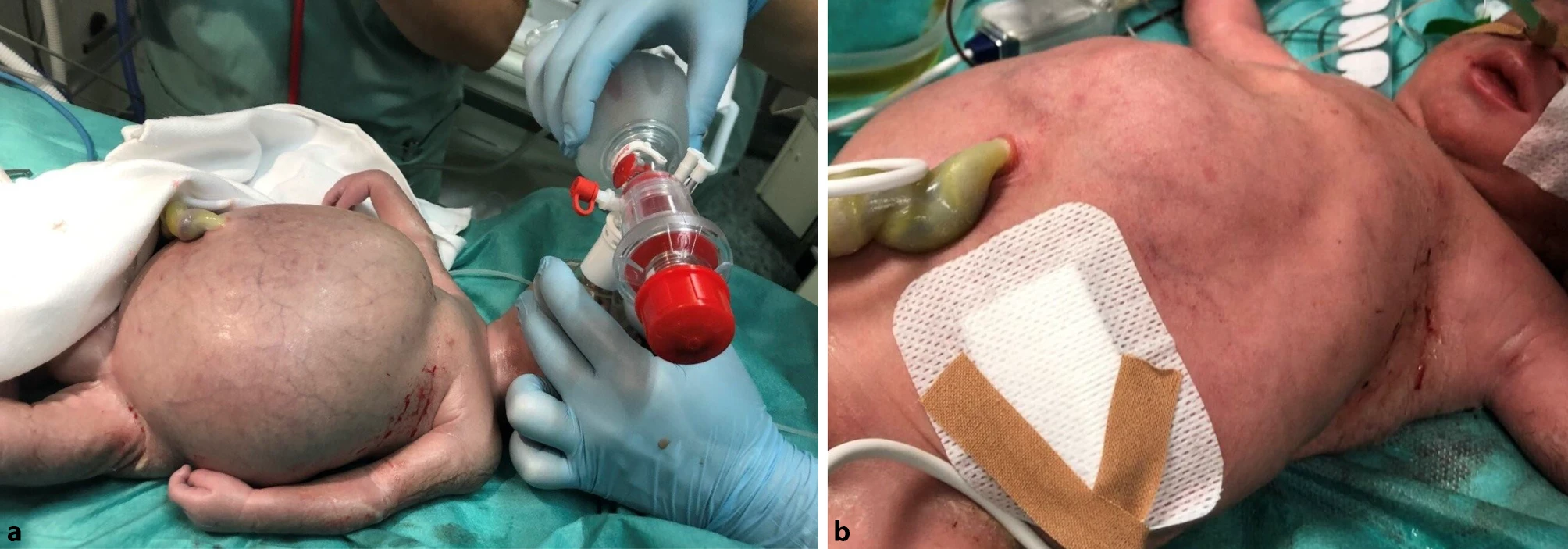
After Birth (Newborn Signs):
In the absence of prophylaxis, symptoms often emerge within the first hours after delivery.
- Pallor and jaundice within the first 24 hours
- Irritability, poor feeding, or unusual sleepiness
- Tachycardia and tachypnea
- Hepatosplenomegaly
- If left untreated,it can lead to Kernicterus (brain damage from severe jaundice)7Myle, A. K., & Al-Khattabi, G. H. (2021). Hemolytic Disease of the Newborn: A Review of Current Trends and Prospects. Pediatric health, medicine and therapeutics, 12, 491–498. https://doi.org/10.2147/PHMT.S327032
What is Kernicterus?
The destruction of fetal RBCs floods the system with unconjugated bilirubin, a yellow pigment that’s toxic at high levels. This buildup can cross the blood-brain barrier and cause permanent brain damage.
Warning signs include:
- Poor feeding
- High-pitched or inconsolable crying
- Floppy limbs (hypotonia) progressing to stiffness (hypertonia)
- Seizures, lethargy, or arching of the back (opisthotonus)
Bilirubin levels above 20 mg/dL in term infants are a medical emergency. Without treatment, kernicterus can cause hearing loss, cerebral palsy, or developmental delays.8Myle, A. K., & Al-Khattabi, G. H. (2021). Hemolytic Disease of the Newborn: A Review of Current Trends and Prospects. Pediatric health, medicine and therapeutics, 12, 491–498. https://doi.org/10.2147/PHMT.S327032
Hydrops Fetalis vs. Erythroblastosis Fetalis
Though often used interchangeably, these two terms refer to distinct, but sometimes connected conditions:
Erythroblastosis fetalis (HDFN) is a blood disorder caused by maternal antibodies attacking fetal red blood cells. This immune response leads to anemia, and in severe cases, can progress to hydrops fetalis.
Hydrops fetalis is a clinical syndrome marked by abnormal fluid accumulation in two or more fetal compartments, such as the abdomen, lungs, skin, or around the heart.9An, X., Wang, J., Zhuang, X., Dai, J., Lu, C., Li, X., & Yan, Y. (2015). Clinical Features of Neonates with Hydrops Fetalis. American journal of perinatology, 32(13), 1231–1239. https://doi.org/10.1055/s-0035-1552934. While severe HDFN can cause hydrops, not all hydrops is immune-mediated.
In fact, thanks to widespread Rh prophylaxis, immune hydrops now accounts for only 10–15% of cases. The majority (85–90%) are non-immune hydrops, often linked to cardiac anomalies, infections, chromosomal disorders, or other conditions.
Diagnosis & Treatment of Erythroblastosis Fetalis
Prenatal Screening:
Diagnosis begins with maternal blood typing and antibody screening in the first trimester of every pregnancy.
- Blood type and Rh status
- Antibody screen (to detect maternal alloimmunization)
If the mother is Rh-negative:
- The father’s Rh status is checked.
- Father Rh-negative? The fetus cannot be Rh-positive → no risk.
- Father Rh-positive or unknown? The fetus may be at risk → Close monitoring.
- Remember that an unsensitized (with a negative antibody screen) Rh-negative mother is always a candidate for Rh immunoprophylaxis (anti-D immunoglobulin), given at 28 weeks and within 72 hours after delivery.
Antibody Monitoring:
If maternal alloantibodies (especially anti-D) are present:
- Antibody titers are monitored regularly (usually every 2–4 weeks).
- If titers rise above a critical threshold (commonly 1:16), fetal monitoring is intensified.
Fetal Monitoring:
To evaluate fetal anemia:
- Doppler ultrasound is used to measure middle cerebral artery peak systolic velocity (MCA-PSV).10Myle, A. K., & Al-Khattabi, G. H. (2021). Hemolytic Disease of the Newborn: A Review of Current Trends and Prospects. Pediatric health, medicine and therapeutics, 12, 491–498. https://doi.org/10.2147/PHMT.S327032
- Increased flow velocity = decreased blood viscosity = anemia
- It’s non-invasive and remarkably reliable.
Confirmation & Treatment:
If MCA-PSV suggests moderate to severe anemia:
- Diagnosis can be confirmed via cordocentesis (fetal blood sampling via the umbilical vein) to:
- Confirm hemoglobin levels
- Crossmatch for possible intrauterine transfusion (IUT)
IUT is the gold standard for severe fetal anemia:
- Rh-negative, antigen-negative blood is directly transfused to the fetus.
- May be repeated every 1–3 weeks until:
- The fetus is stable
- Or it’s safe to deliver
Delivery:
Timing depends on a balance:
- Fetal lung maturity
- Severity of anemia
- Response to transfusions
Delivery is planned accordingly—sometimes early, often with steroids for lung maturity if preterm.
Postnatal Assessement:
Once the baby is born, if HDFN is suspected or confirmed:
- Direct Coombs test (DAT): Confirms the presence of maternal antibodies attached to neonatal RBCs.
- Complete blood count (CBC) and reticulocyte count: Assesses anemia and marrow response
- Serum bilirubin levels: Evaluates the severity of jaundice
- Peripheral smear: Reveals nucleated RBCs and polychromasia
- ABO and Rh typing of both mother and infant should be reviewed.
Management & Treatment:
In mild cases of jaundice:
- Phototherapy: First-line treatment for hyperbilirubinemia. It converts unconjugated bilirubin into excretable isomers.11Myle, A. K., & Al-Khattabi, G. H. (2021). Hemolytic Disease of the Newborn: A Review of Current Trends and Prospects. Pediatric health, medicine and therapeutics, 12, 491–498. https://doi.org/10.2147/PHMT.S327032
In severe cases, though, exchange transfusion and IVIG are urgently needed.
- Exchange Transfusion: For severe anemia or rapidly rising bilirubin. It replaces antibody-coated cells and lowers bilirubin quickly
- Intravenous immunoglobulin (IVIG): May reduce hemolysis by blocking Fc receptors in the neonatal reticuloendothelial system. This prevents immune cells from recognizing and destroying red cells that are tagged with maternal antibodies.
- Supportive care, including hydration and feeding support
Emerging Treatments:
Nipocalimab, a monoclonal antibody that blocks IgG transfer across the placenta shows promise in reducing the need for intrauterine transfusions.12Moise, K. J., Jr, Ling, L. E., Oepkes, D., Tiblad, E., Verweij, E. J. T. J., Lopriore, E., Smoleniec, J., Sachs, U. J., Bein, G., Kilby, M. D., Miller, R. S., Devlieger, R., Audibert, F., Emery, S. P., Markham, K., Norton, M. E., Ocón-Hernández, O., Pandya, P., Pereira, L., Silver, R. M., … UNITY Study Group (2024). Nipocalimab in Early-Onset Severe Hemolytic Disease of the Fetus and Newborn. The New England journal of medicine, 391(6), 526–537. https://doi.org/10.1056/NEJMoa2314466 Advances in noninvasive prenatal testing (NIPT) and improvements in transfusion tools are also making care safer and more targeted.
Prevention of Erythroblastosis Fetalis
The most effective strategy is preventing maternal sensitization using Rh immunoglobulin (RhIg).
- Routine prophylaxis: Given at 28 weeks of gestation and within 72 hours postpartum13 V, Vadakekut ES, Avulakunta ID. Hemolytic Disease of the Fetus and Newborn. [Updated 2025 Jan 22]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK557423/
- After sensitizing events: Following miscarriage, trauma, invasive procedures (amniocentesis, chorionic villus sampling), or abdominal trauma
- Maternal counseling: Emphasizing the importance of completing prophylaxis and seeking care after any pregnancy complications
Cases still occur due to missed or delayed doses, late prenatal care, or sensitization to non-D Rh antigens (e.g., anti-c, anti-E), which RhIg doesn’t cover.
Prognosis & Follow Up
Erythroblastosis fetalis has become a preventable and treatable condition in much of the world. With proper prevention and treatment:
- Survival rates exceed 90–95%
- Early detection and management yield excellent outcomes
- Infants who experienced severe disease require:
- Serial monitoring for late anemia and jaundice
- Hearing assessment (kernicterus risk)
- Developmental follow-up if severe hyperbilirubinemia occurred
- For sensitized mothers, all future pregnancies require close monitoring regardless of fetal blood type.
Conclusion
Once a devastating diagnosis, Erythroblastosis fetalis has now changed to an easily preventable condition. The widespread use of Rh immunoglobulin represents one of obstetric medicine’s greatest success stories, dramatically reducing both incidence and mortality.
Recent discoveries like placental antibody-blocking therapies and noninvasive genotyping signals a shift toward more precise, less invasive care. With new clinical tools in hand, the focus has now turned to access, equity, and global implementation: ensuring that every fetus at risk receives timely, effective intervention, wherever they are.
Refrences
- 1Moinuddin, I., Fletcher, C., & Millward, P. (2019). Prevalence and specificity of clinically significant red cell alloantibodies in pregnant women – a study from a tertiary care hospital in Southeast Michigan. Journal of blood medicine, 10, 283–289. https://doi.org/10.2147/JBM.S214118
- 2Ohto, H., Denomme, G. A., Ito, S., Ishida, A., Nollet, K. E., & Yasuda, H. (2020). Three non-classical mechanisms for anemic disease of the fetus and newborn, based on maternal anti-Kell, anti-Ge3, anti-M, and anti-Jra cases. Transfusion and apheresis science : official journal of the World Apheresis Association : official journal of the European Society for Haemapheresis, 59(5), 102949. https://doi.org/10.1016/j.transci.2020.102949
- 3Gabbay, J. M., Agneta, E. M., Turkington, S., Bajaj, B. M., Sinha, B., & Geha, T. (2023). Rates of phototherapy among ABO-incompatible newborns with a negative direct antiglobulin test. Journal of perinatology : official journal of the California Perinatal Association, 43(11), 1357–1362. https://doi.org/10.1038/s41372-023-01650-3
- 4Zipursky A, Bhutani VK. Impact of Rhesus disease on the global problem of bilirubin-induced neurologic dysfunction. Semin Fetal Neonatal Med. 2015 Feb;20(1):2-5. doi: 10.1016/j.siny.2014.12.001. Epub 2015 Jan 9. PMID: 25582277.
- 5Myle, A. K., & Al-Khattabi, G. H. (2021). Hemolytic Disease of the Newborn: A Review of Current Trends and Prospects. Pediatric health, medicine and therapeutics, 12, 491–498. https://doi.org/10.2147/PHMT.S327032
- 6Myle, A. K., & Al-Khattabi, G. H. (2021). Hemolytic Disease of the Newborn: A Review of Current Trends and Prospects. Pediatric health, medicine and therapeutics, 12, 491–498. https://doi.org/10.2147/PHMT.S327032
- 7Myle, A. K., & Al-Khattabi, G. H. (2021). Hemolytic Disease of the Newborn: A Review of Current Trends and Prospects. Pediatric health, medicine and therapeutics, 12, 491–498. https://doi.org/10.2147/PHMT.S327032
- 8Myle, A. K., & Al-Khattabi, G. H. (2021). Hemolytic Disease of the Newborn: A Review of Current Trends and Prospects. Pediatric health, medicine and therapeutics, 12, 491–498. https://doi.org/10.2147/PHMT.S327032
- 9An, X., Wang, J., Zhuang, X., Dai, J., Lu, C., Li, X., & Yan, Y. (2015). Clinical Features of Neonates with Hydrops Fetalis. American journal of perinatology, 32(13), 1231–1239. https://doi.org/10.1055/s-0035-1552934
- 10Myle, A. K., & Al-Khattabi, G. H. (2021). Hemolytic Disease of the Newborn: A Review of Current Trends and Prospects. Pediatric health, medicine and therapeutics, 12, 491–498. https://doi.org/10.2147/PHMT.S327032
- 11Myle, A. K., & Al-Khattabi, G. H. (2021). Hemolytic Disease of the Newborn: A Review of Current Trends and Prospects. Pediatric health, medicine and therapeutics, 12, 491–498. https://doi.org/10.2147/PHMT.S327032
- 12Moise, K. J., Jr, Ling, L. E., Oepkes, D., Tiblad, E., Verweij, E. J. T. J., Lopriore, E., Smoleniec, J., Sachs, U. J., Bein, G., Kilby, M. D., Miller, R. S., Devlieger, R., Audibert, F., Emery, S. P., Markham, K., Norton, M. E., Ocón-Hernández, O., Pandya, P., Pereira, L., Silver, R. M., … UNITY Study Group (2024). Nipocalimab in Early-Onset Severe Hemolytic Disease of the Fetus and Newborn. The New England journal of medicine, 391(6), 526–537. https://doi.org/10.1056/NEJMoa2314466
- 13V, Vadakekut ES, Avulakunta ID. Hemolytic Disease of the Fetus and Newborn. [Updated 2025 Jan 22]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK557423/