Anemia is one of the most common laboratory abnormalities observed in pediatric practice. It can arise from three primary mechanisms: decreased production of red blood cells (RBCs), increased loss of RBCs, or premature destruction of RBCs, known as hemolysis. In some conditions, these mechanisms can occur concurrently. Hemolytic anemias are characterized by a reduced lifespan of erythrocytes due to premature destruction. Understanding the underlying pathophysiology of this condition is crucial for accurate diagnosis and effective treatment.
What is Hemolytic Anemia?
This is a disorder in which the red blood cells are destroyed faster than your body can replace them. The bone marrow cannot compensate for the loss, resulting in decreased hemoglobin in your body, causing anemia. This causes insufficient oxygen to be transported to the body’s tissues. Symptoms of anemia depend upon the degree and cause of hemolysis in your body.1Baldwin, C., Pandey, J., & Olarewaju, O. (2023). Hemolytic Anemia. In StatPearls. StatPearls Publishing.
Anemia develops due to less production of hemoglobin in your body. A hemoglobin value of less than 13 g/dl in men and less than 12 g/dl in women is considered anemia. Anemia has three types based on the mean corpuscular volume (MCV), which is the size of your red blood cells.
- Normocytic anemia- MCV normal ( 80-100 fL )
- Microcytic anemia- MCV less than 80 fL
- Macrocytic anemia- MCV more than 100 fL
Hemolytic is a type of normocytic anemia in which the size of your red blood cells remains the same, but due to the destruction, the amount of hemoglobin is reduced in your blood.
Types of Hemolytic Anemia
It can be classified as either inherited or acquired:
Inherited Hemolytic Anemia:
This is also called intrinsic hemolytic anemia. It occurs due to genetic mutations that affect the production or function of RBCs. As a result, these cells are destroyed earlier than normal. Examples include conditions like sickle cell anemia and hereditary spherocytosis.
Acquired Hemolytic Anemia:
Unlike inherited forms, acquired or extrinsic hemolytic anemia develops later in life and is not present at birth. This form is caused by factors external to the red blood cells, such as antibodies from autoimmune disorders, burns, or certain medications. In these cases, the RBCs are typically healthy when produced by the bone marrow but are later destroyed in the bloodstream or prematurely trapped and recycled in the spleen.
Pathophysiology of Hemolytic Anemia
This condition occurs due to some inherited defect or any acquired factor that destroys your red blood cells. Any red blood cell membrane defect results in hemolysis, such as hereditary spherocytosis and paroxysmal nocturnal hemoglobinuria. Sometimes, deficiency of specific enzymes like pyruvate kinase and G6PD also destroys your RBCs. Hemolysis also occurs when a defect is present in the standard structure of hemoglobin, such as sickle cell anemia.
In hemolysis, the destruction of red blood cells can occur either intravascularly or extravascularly. Extravascular hemolysis means red blood cells are destroyed outside the bloodstream, primarily in the spleen or liver. In this process, macrophages in the spleen and liver engulf and break down aged or damaged RBCs, leading to increased bilirubin levels and symptoms like jaundice.2Medscape. (n.d.). Overview of hemolytic anemia. Retrieved from https://emedicine.medscape.com/article/201066-overview?form=fpf#a
Some examples of hemolytic anemia with extravascular hemolysis are:
- Autoimmune hemolytic anemia
- Hereditary spherocytosis
- Sickle cell disease
In intravascular hemolysis, red blood cells are destroyed directly within the blood vessels. Common causes include mechanical damage (e.g., from artificial heart valves), infections (e.g., malaria), and certain autoimmune reactions.
Hemolytic Anemias with intravascular hemolysis are:
- Glucose 6 phosphate deficiency (In this, red blood cells are more susceptible to hemolysis when exposed to certain triggers and medications)
- Pyruvate kinase deficiency (impairs energy production in RBCs, leading to their premature destruction)
- Paroxysmal nocturnal hemoglobinuria (PNH)
- Prosthetic valves
- Transfusion of ABO-incompatible blood
When RBCs break apart in the bloodstream, hemoglobin is released directly into the plasma, leading to symptoms such as hemoglobinemia and hemoglobinuria (hemoglobin in urine). Moreover, hemolysis also increases the risk of gallstones.3van Wijk, R., & Bianchi, P. (2020). Editorial: Pathophysiology of Rare Hemolytic Anemias. Frontiers in physiology, 11, 601746. https://doi.org/10.3389/fphys.2020.601746
Causes of Hemolytic Anemia
This condition develops either due to extrinsic factors or intrinsic causes.
Intrinsic Causes:
Defects present in the red blood cell itself are the reason for intrinsic hemolysis. These are:
Membrane Defects
Hemolytic anemias in which there is a membrane defect leading to the destruction of your red blood cells are hereditary spherocytosis and paroxysmal nocturnal hemoglobinuria (PNH). In hereditary spherocytosis, defects in specific proteins (ankyrin, spectrin) cause damage to the membranes and produce small and rounded red blood cells. These defective cells are destroyed by your spleen (extravascular hemolysis) and cause enlargement of the spleen. In PNH, PIGA gene mutation causes RBC destruction in your body through the complement system, leading to hemolysis, especially at night.
Enzyme Deficiencies
G6PD and pyruvate kinase deficiency lead to this anemia. Deficiency of G6PD is an X-linked disorder and causes your red blood cells to go under oxidative stress, producing bite cells. Drugs like sulfa drugs and antimalarials also lead to G6PD deficiency. Pyruvate Kinase deficiency (autosomal recessive) makes your RBCs rigid due to decreased ATP. These rigid RBCs undergo extravascular hemolysis by passing through your spleen and producing burr cells.
Hemoglobinopathies
A defect in the typical structure of hemoglobin causes hemolysis, such as sickle cell anemia. In sickle cell anemia, point mutation causes valine to replace the glutamic acid in the beta-globin chain of your hemoglobin. It disturbs the normal shape of your red blood cells and makes them spherical.
Extrinsic Causes:
Hemolytic anemias in which an extrinsic factor affects your red blood cells are:
Autoimmune Hemolytic Anemia
In autoimmune hemolytic anemia, antibodies like IgM or IgG are the reason for red blood cell destruction, and this destruction produces spherocytes.
Micro & Macroangiopathic Anemias
Obstruction of your vessels due to DIC, TTP, or SLE destroys the red blood cells when they pass through a narrow vessel, causing microangiopathic hemolytic anemia. If you have a prosthetic heart valve, the mechanical destruction of RBCs leads to macroangiopathic hemolytic anemia.
Infections
Some infections, such as malaria or Babesia, may also cause hemolytic anemia by destroying your RBCs.
Anemia due to certain Drugs
Antibiotics (penicillin, cephalosporins), Nonsteroidal anti-inflammatory drugs, immunotherapy, and chemotherapy (for treating cancers) cause hemolysis and produce bite cells. These drugs can cause both extravascular and intravascular hemolysis.4Guillaud, C., Loustau, V., & Michel, M. (2012). Hemolytic anemia in adults: main causes and diagnostic procedures. Expert review of hematology, 5(2), 229–241. https://doi.org/10.1586/ehm.12.3
Histopathology of Hemolytic Anemia
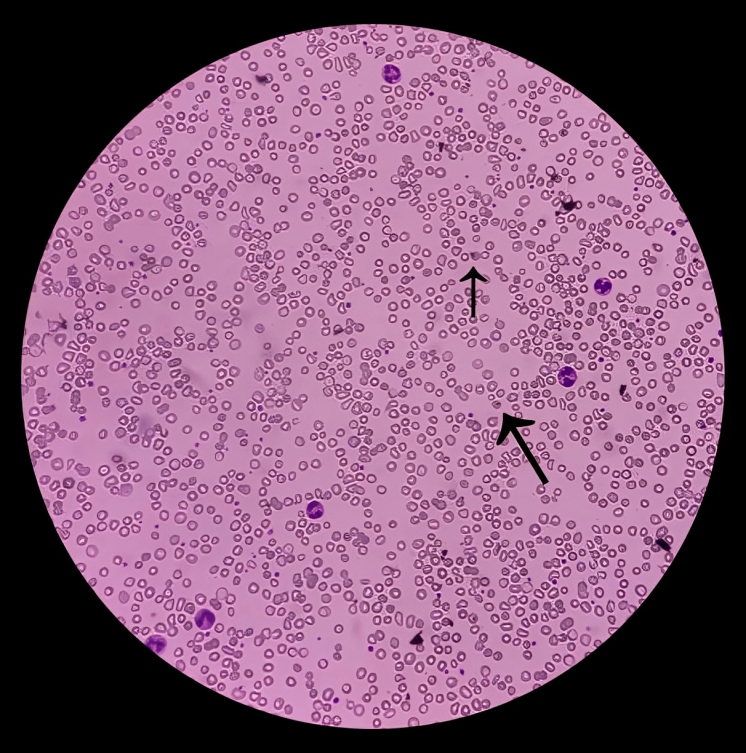
Histopathology plays a crucial role in the diagnosis of hemolytic anemia. Depending upon the cause of hemolytic anemia, there is either a change in the size or shape of your red blood cells, which makes it effortless for a physician to diagnose hemolytic anemia. In the case of oxidative stress, such as in G6PD deficiency, a blood smear shows typical bite cells and Heinz bodies. There is the presence of spherocytes in hereditary spherocytosis, schistocytes in hemolytic anemia, and sickle cells in sickle cell anemia. Therefore, the shape of RBC assists in diagnosing the cause of hemolytic anemia.
Symptoms of Hemolytic Anemia
Symptoms of hemolytic anemia vary from individual to individual. People with mild anemia often do not have any signs or symptoms. In most cases, physicians diagnose anemia during laboratory tests for some other diseases. However, if anemia is severe, you may experience the following symptoms:5Phillips, J., & Henderson, A. C. (2018). Hemolytic Anemia: Evaluation and Differential Diagnosis. American Family Physician, 98(6), 354–361.
- Fatigue
- Shortness of breath
- Tachycardia (increased heart rate )
- Tachypnea (increased respiratory rate)
- Jaundice
- Dark urine
- Abdominal pain if gallstones are present
Diagnosis of Hemolytic Anemia
Your physician can easily interpret anemia by taking a proper history of symptoms. Physical signs like yellowness of eyes and palms also aid in making a diagnosis. However, following laboratory tests are beneficial for ensuring a diagnosis.
Complete Blood Cell Count:
A complete blood count shows a decreased amount of hemoglobin in your blood. It also indicates the reduced level of other cells like platelets and white blood cells. CBC of hemolytic anemia due to SLE, thrombotic thrombocytopenic purpura, HUS, and microangiopathic hemolytic anemia shows a decreased level of platelets in your blood.
A complete blood count also reveals the values of MCV and MCH, indicating whether anemia is either microcytic or macrocytic. The destruction of red blood cells produces cells with different widths, leading to increased red blood cell distribution width (RDW).
Peripheral Blood Smear:
Peripheral blood smears enable your physician to discover the cause of hemolytic anemia as they demonstrate the abnormal shape of RBCs due to destruction. Some examples are spherocytes in hereditary spherocytosis and schistocytes in HUS, DIC, and TTP.
LDH & Serum Haptoglobin Level:
During the destruction of red blood cells, the level of LDH increases in the blood as it is present intracellularly. Haptoglobin binds with free hemoglobin, and due to destruction, all the hemoglobin gets occupied by haptoglobin, leading to a decreased level of haptoglobin.
Indirect Bilirubin:
The destruction of red blood cells does not allow the complete removal of unconjugated bilirubin, which causes its increased level in the blood.
Some other Tests:
Some tests are specific to certain types of hemolytic anemia. These are:6Facp, S. N. M. M. (n.d.). Hemolytic anemia workUp: approach considerations, complete blood cell count, peripheral blood smear. https://emedicine.medscape.com/article/201066-workup
- Direct antiglobulin test for autoimmune hemolytic anemia
- Urine hemosiderin test (hemosiderin increases if you have intravascular hemolysis )
- Cold agglutinin titer
- Glucose 6 phosphate dehydrogenase screening in G6PD deficiency
- Sickle cell screen for sickle cell anemia
Treatment of Hemolytic Anemia:
The treatment plan for anemia highly depends upon the severity and cause of anemia. It also depends on your personal preference. For mild anemia, your physician may advise you to take oral medications. Some of the treatment strategies are:
Folic Acid:
It is better to take folic acid tablets in mild cases of anemia to avoid complications. Its use is because hemolysis decreases the amount of folic acid in your body.
Corticosteroids, Rituximab, and IVIG:
These are prescribed explicitly if a patient is diagnosed with autoimmune hemolytic anemia. For patients who are resistant to steroids, the next best option is to take rituximab, especially in warm autoimmune hemolytic anemia. You can also go for intravenous immunoglobulins, but the results are not promising.
Blood Transfusion:
You should only choose blood transfusion to treat anemia when all other methods are unsuccessful. Proper cross-matching and typing before the transfusion are crucial to avoid acute and hyper-acute transfusion reactions. The infusion rate of packed RBCs should be slow to prevent further destruction of red blood cells.
One of the most common complications that you may encounter due to multiple transfusions is iron overload. Chelation therapy with deferoxamine deferasirox, or deferiprone, treats iron overload as it removes the excessive iron from your blood and liver.
Erythropoietin Therapy:
People reluctant to do blood transfusion to treat anemia can choose erythropoietin therapy as it decreases the need for transfusion. However, it is much more expensive than blood transfusion. Erythropoietin therapy is used in children with kidney failure and hereditary spherocytosis, patients having sickle cell anemia along with kidney failure, and autoimmune hemolytic anemia with reticulocytopenia (decreased reticulocytes).
Removal of the Spleen:
Splenectomy helps to reduce extravascular hemolysis in patients with hereditary spherocytosis and autoimmune hemolytic anemia. There is a high risk of sepsis within two years of splenectomy by certain bacteria, so it is beneficial for you to get immunized after the operation.7Coon W. W. (1985). Splenectomy in the treatment of hemolytic anemia. Archives of surgery (Chicago, Ill. : 1960), 120(5), 625–628. https://doi.org/10.1001/archsurg.1985.01390290099017
Iron Therapy:
Generally, iron therapy is not recommended for the treatment of this condition. However, hemolytic anemia with severe intravascular hemolysis causes a significant decrease in your body’s iron stores. In such cases, iron therapy is recommended. You better check your iron levels before starting iron therapy to avoid overload.8Maakaron, J. E., MD. (n.d.). Anemia Treatment & Management: approach considerations, transfusion, iron supplementation. https://emedicine.medscape.com/article/198475-treatment
Difference between Cold & Warm Hemolytic Anemia
Both warm and cold autoimmune hemolytic anemias are a type of normocytic anemia with average Mean corpuscular volume, and both exhibit a positive Coombs test. However, there are some differences.
Warm hemolytic anemia develops due to IgG-mediated complement activation that leads to extravascular hemolysis—the extravascular hemolysis results in the formation of spherocytes. Warm hemolytic anemia is seen in systemic lupus erythematosus (SLE), chronic lymphocytic leukemia (CLL), and certain drugs like alpha-methyldopa. About 5-10% of patients with CLL may develop warm autoimmune hemolytic anemia (AIHA) during their disease course. Steroids, rituximab, and even splenectomy are used to treat warm hemolytic anemia.
Cold hemolytic anemia has IgM-mediated complement activation that causes agglutination of your RBCs. With exposure to cold, extravascular hemolysis occurs, exhibiting symptoms of pain and blueness of your fingers and toes. It is seen in patients with CLL and infectious mononucleosis. Cold AIHA is less commonly associated with CLL than warm AIHA but can still occur. Cold hemolytic anemia is prevented by avoiding cold weather and taking rituximab.9Brazel, D., Eid, T., & Harding, C. (2021). Warm and Cold Autoimmune Hemolytic Anemia in the Setting of COVID-19 Disease. Cureus, 13(9), e18127. https://doi.org/10.7759/cureus.18127
What should you eat if you have hemolytic anemia?
For patients with mild anemia that can be treated without transfusion, a healthy diet is recommended to treat anemia. A diet low in iron is advisable to avoid iron overload. You should prevent iron cereals and red meat. Moreover, your physician may prophylactically prescribe you folic acid tablets to prevent its deficiency in your body. Add healthy things to your diet, like fresh fruits, leafy vegetables, and eggs.
Prognosis of Hemolytic Anemia
The prognosis depends upon its cause, early diagnosis, and management. If it is properly managed at an early stage by medication and diet, the prognosis improves. The risk of mortality increases in patients with additional morbidities like CKD or malignancy.
People with sickle cell anemia have a higher chance of mortality as it causes stroke if hemoglobin decreases to less than 8g/dl.10Hansen, D. L., Möller, S., & Frederiksen, H. (2022). Survival in autoimmune hemolytic anemia remains poor, results from a nationwide cohort with 37 years of follow-up. European journal of hematology, 109(1), 10–20. https://doi.org/10.1111/ejh.13764
Conclusion
In conclusion, hemolytic anemia has multiple causes, and all of them destroy your red blood cells in one way or another. Destruction of red blood cells reduces the hemoglobin levels in your body. Get your complete and peripheral blood count, LDH levels, and serum haptoglobin level checked as soon as possible if you feel symptoms like fatigue, weakness, or shortness of breath. Take folic acid tablets, eat a healthy diet, and take iron supplements. Blood transfusion is a reasonable treatment option if the hemoglobin level is too low. This condition has a good prognosis if it is early diagnosed and well-treated.
Refrences
- 1Baldwin, C., Pandey, J., & Olarewaju, O. (2023). Hemolytic Anemia. In StatPearls. StatPearls Publishing.
- 2Medscape. (n.d.). Overview of hemolytic anemia. Retrieved from https://emedicine.medscape.com/article/201066-overview?form=fpf#a
- 3van Wijk, R., & Bianchi, P. (2020). Editorial: Pathophysiology of Rare Hemolytic Anemias. Frontiers in physiology, 11, 601746. https://doi.org/10.3389/fphys.2020.601746
- 4Guillaud, C., Loustau, V., & Michel, M. (2012). Hemolytic anemia in adults: main causes and diagnostic procedures. Expert review of hematology, 5(2), 229–241. https://doi.org/10.1586/ehm.12.3
- 5Phillips, J., & Henderson, A. C. (2018). Hemolytic Anemia: Evaluation and Differential Diagnosis. American Family Physician, 98(6), 354–361.
- 6Facp, S. N. M. M. (n.d.). Hemolytic anemia workUp: approach considerations, complete blood cell count, peripheral blood smear. https://emedicine.medscape.com/article/201066-workup
- 7Coon W. W. (1985). Splenectomy in the treatment of hemolytic anemia. Archives of surgery (Chicago, Ill. : 1960), 120(5), 625–628. https://doi.org/10.1001/archsurg.1985.01390290099017
- 8Maakaron, J. E., MD. (n.d.). Anemia Treatment & Management: approach considerations, transfusion, iron supplementation. https://emedicine.medscape.com/article/198475-treatment
- 9Brazel, D., Eid, T., & Harding, C. (2021). Warm and Cold Autoimmune Hemolytic Anemia in the Setting of COVID-19 Disease. Cureus, 13(9), e18127. https://doi.org/10.7759/cureus.18127
- 10Hansen, D. L., Möller, S., & Frederiksen, H. (2022). Survival in autoimmune hemolytic anemia remains poor, results from a nationwide cohort with 37 years of follow-up. European journal of hematology, 109(1), 10–20. https://doi.org/10.1111/ejh.13764