Loiasis, also known as African eye worm, is a filarial nematode infection. Loa Loa, the causative agent of this infectious disease, is a filarial pathogen. It is transmitted through the bite of tabanid flies of the Chrysops genus, commonly known as deer flies or mango flies. The infection manifests with localized angioedema, known as Calabar swellings, and the migration of adult worms beneath the skin and across the conjunctiva. Diagnosis is confirmed by detecting microfilariae in peripheral blood or directly observing worms moving across the eye. Treatment typically involves diethylcarbamazine (DEC) to eliminate the parasites.
Prevalence of Loiasis
The disease is endemic to the rainforests of West and Central Africa, particularly in regions of Cameroon, Gabon, Equatorial Guinea, the Republic of the Congo, and the Democratic Republic of the Congo. More than 20 million people become chronically infected in the medium to high transmission regions. Loiasis poses a substantial disease burden on communities. The measured morbidity is as high as 400 per 100,000 residents. The population-attributable fraction of death in highly endemic areas due to loiasis is about 14.5%.1Ramharter M, Butler J, Mombo-Ngoma G, Nordmann T, Davi SD, Manego RZ. The African eye worm: current understanding of the epidemiology, clinical disease, and treatment of loiasis. The Lancet Infectious Diseases. 2024;24(3):e165-e78.
Researchers are still struggling to quantify the survival rate for loiasis universally. This is due to varying factors such as individual health conditions and geographical location. Loiasis is highly prevalent and estimated to be the third most common reason for medical consultation. However, it is still considered a relatively benign disease. Loiasis is still absent from the list of prioritized neglected tropical diseases of the World Health Organization.2Buell KG, Whittaker C, Chesnais CB, Jewell PD, Pion SD, Walker M, et al., editors. Atypical clinical manifestations of loiasis and their relevance for endemic populations. Open Forum Infectious Diseases; 2019: Oxford University Press US. Public health officials can reduce the burden of this condition. This can be through expanding the use of diagnostic tests, control of Chrysops spp vectors, and anthelmintic treatment.3Jacobsen KH, Andress BC, Bhagwat EA, Bryant CA, Chandrapu VR, Desmonts CG, et al. A call for loiasis to be added to the WHO list of neglected tropical diseases. The Lancet Infectious Diseases. 2022;22(10):e299-e302.
Structure & Characteristics of the L. Loa
The worm has a very simple structure consisting of a head, a body, and a blunt tail. Juveniles have the same structural appearance as adult worms except for their size. The outer body comprises a cuticle, three layers of collagen, and some other components. This structure aids in protecting the nematode while it is in the digestive system of its host. Adult male worms are 30 to 35 mm in length and 0.4 mm in width. Adult female worms are 0.4 mm in width and 50 to 70 mm long. The adult L. loa live in the subcutaneous tissues of the host body and migrate freely. People often see the worms moving under the conjunctiva, named African eye worms.4Burton J. Bogitsh Phd CECP, Thomas N. Oeltmann Phd. Chapter 17 – Blood and Tissue Nematodes. fourth Edition ed2013.
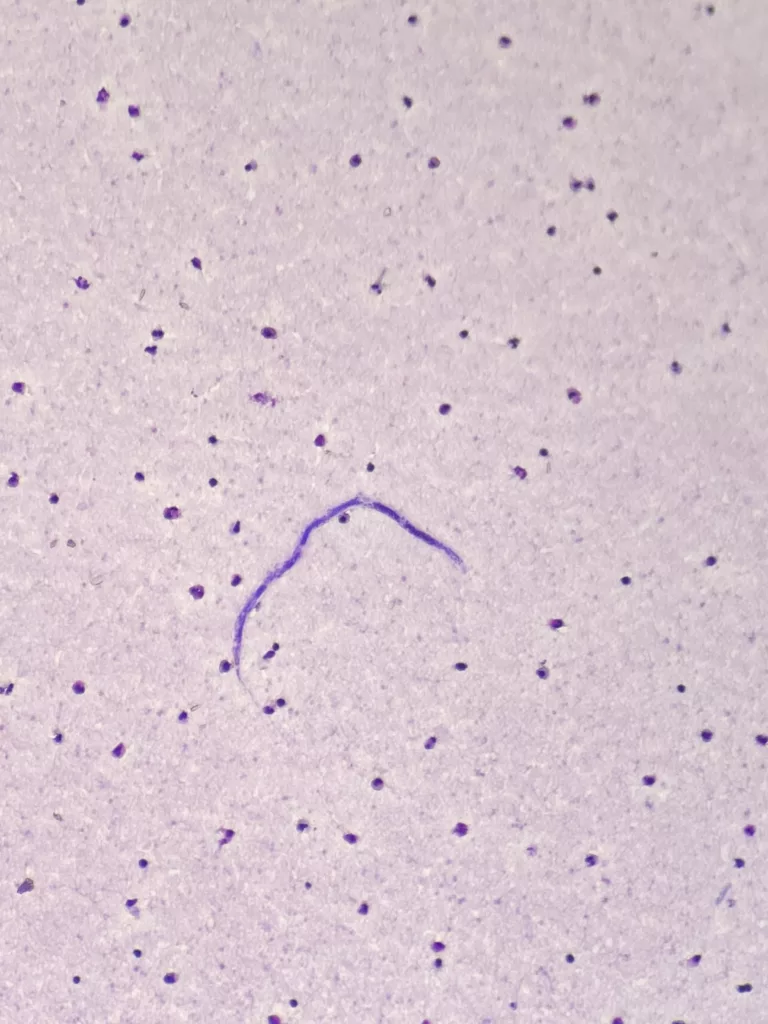
Causes of Loiasis
A bite from the infected deer fly, which carries the L. loa larvae, can cause loiasis. Deer flies are blood-sucking insects that are most active during the daytime, typically between 10 AM and 2 PM. Humans serve as the primary reservoir for this parasite. When a deer fly bites an infected person, it ingests microfilariae (immature worm forms) present in the blood. Inside the fly, these microfilariae develop into infective larvae. When the infected fly bites another person, it deposits the larvae through the bite wound, leading to a new infection.
Life Cycle of L. loa
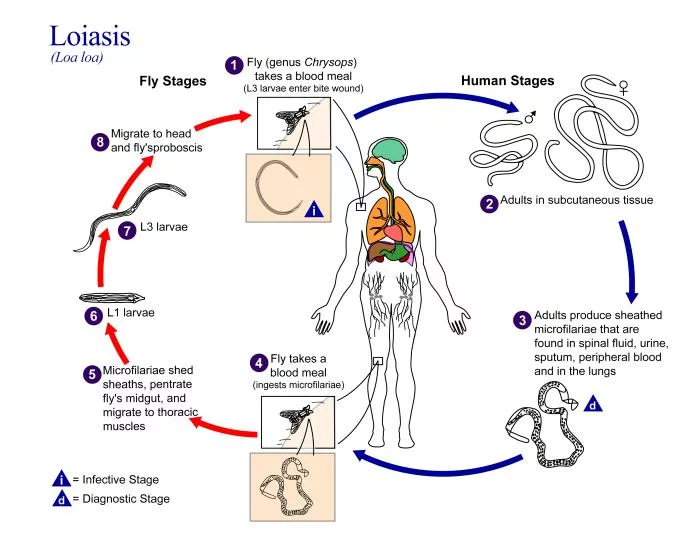
The life cycle of L. loa involves two hosts, humans and deer flies. Primarily two species of tabanid flies, C. dimidiate and C. silacea transmit L. loa in humans.5Whittaker C, Walker M, Pion SD, Chesnais CB, Boussinesq M, Basáñez M-G. The population biology and transmission dynamics of Loa loa. Trends in parasitology. 2018;34(4):335-50.
Transmission Phase:
The cycle begins when a female deer fly bites an individual harboring L. loa microfilariae. The fly ingests larvae present in the blood of the human host. The adult L. loa resides in the layers of the loose connective tissues under the skin. It may also be present between the fascial layers on the top of the somatic muscles, where they reproduce and release microfilariae into the bloodstream.
Development in the Vector:
Once inside the deer fly, the ingested microfilariae migrate to the thoracic muscles, where they develop into third-stage infective larvae (L3) over a period of approximately 10 to 12 days. The duration of development is the extrinsic incubation period (EIP). This period varies with the ambient temperature. It can take as much as three to four weeks in the cooler regions. When the larvae reach maturity, they migrate to the proboscis of the fly, positioning themselves for transmission to a new human host during the fly’s next blood meal.
Development in the Host:
After inoculation into the humans, the L3 larvae do a third molt to develop into fourth-stage larvae (L4). After the third molt, the larvae undergo a fourth and final molt to become the adult worm. These adult worms live in several areas under the connective tissues.
Reproduction:
After development and being sexually mature, females produce microfilariae. These microfilariae accumulate periodically in the peripheral blood. From there, the vectors ingest them during a blood meal. However, the developmental processes vary in different model organisms. In monkeys, transitioning from L3 to L4 takes about 16 to 20 days. In rodents, this transition happens in around 20 days. Also, healthcare providers are unable to detect microfilariae in the peripheral blood until five to six months. This implies further delay in reproduction or their release into the bloodstream from the lungs. The process of reproduction and infection to a new host can occur continuously for many years. The females can produce thousands of microfilariae daily.6Whittaker C, Walker M, Pion SD, Chesnais CB, Boussinesq M, Basáñez M-G. The population biology and transmission dynamics of Loa loa. Trends in parasitology. 2018;34(4):335-50.
Pathogenesis
Adult Loa loa worms migrate through the subcutaneous tissues and may cross into the subconjunctival tissues of the eye, leading to the characteristic visible migration of the worm, which is a hallmark symptom of loiasis. This migration causes localized inflammatory reactions, known as Calabar swellings, which result from the immune system’s response to the parasite’s movement. The immune response to L. loa is complex and exhibits a dual pattern. Approximately 30% of infected individuals are microfilaremic (having detectable microfilariae in the blood), while 70% are amicrofilaremic (lacking detectable microfilariae despite infection). Microfilaremic people have a strong proliferative response after stimulation with L3 larva antigen or adult microfilaria. On the other hand, amicrofilaremic individuals have a low response. The induction of cytokine production is higher in amicrofilaremic patients than in microfilaremic patients.7Paul AJ. Loa loa Pathogenesis in Humans. Human Emerging and Re‐emerging Infections: Viral and Parasitic Infections. 2015:441-52.
Healthcare providers also observe elevated levels of some specific immunoglobulins, particularly IgE and IgG4 in patients suffering from loiasis. These antibodies indicate a complex immune response that includes both allergic reactions and attempts to control parasitic infections.8Paul AJ. Loa loa Pathogenesis in Humans. Human Emerging and Re‐emerging Infections: Viral and Parasitic Infections. 2015:441-52.
Clinical Manifestations of Loiasis
The clinical manifestations of loiasis vary from mild to severe. The most common symptoms include:
• The migration of adult Loa loa worms across the eye, which is a hallmark feature of the infection. There is visible worm movement in the inferior bulbar conjunctiva, often appearing as a white, thread-like structure under a slit lamp.
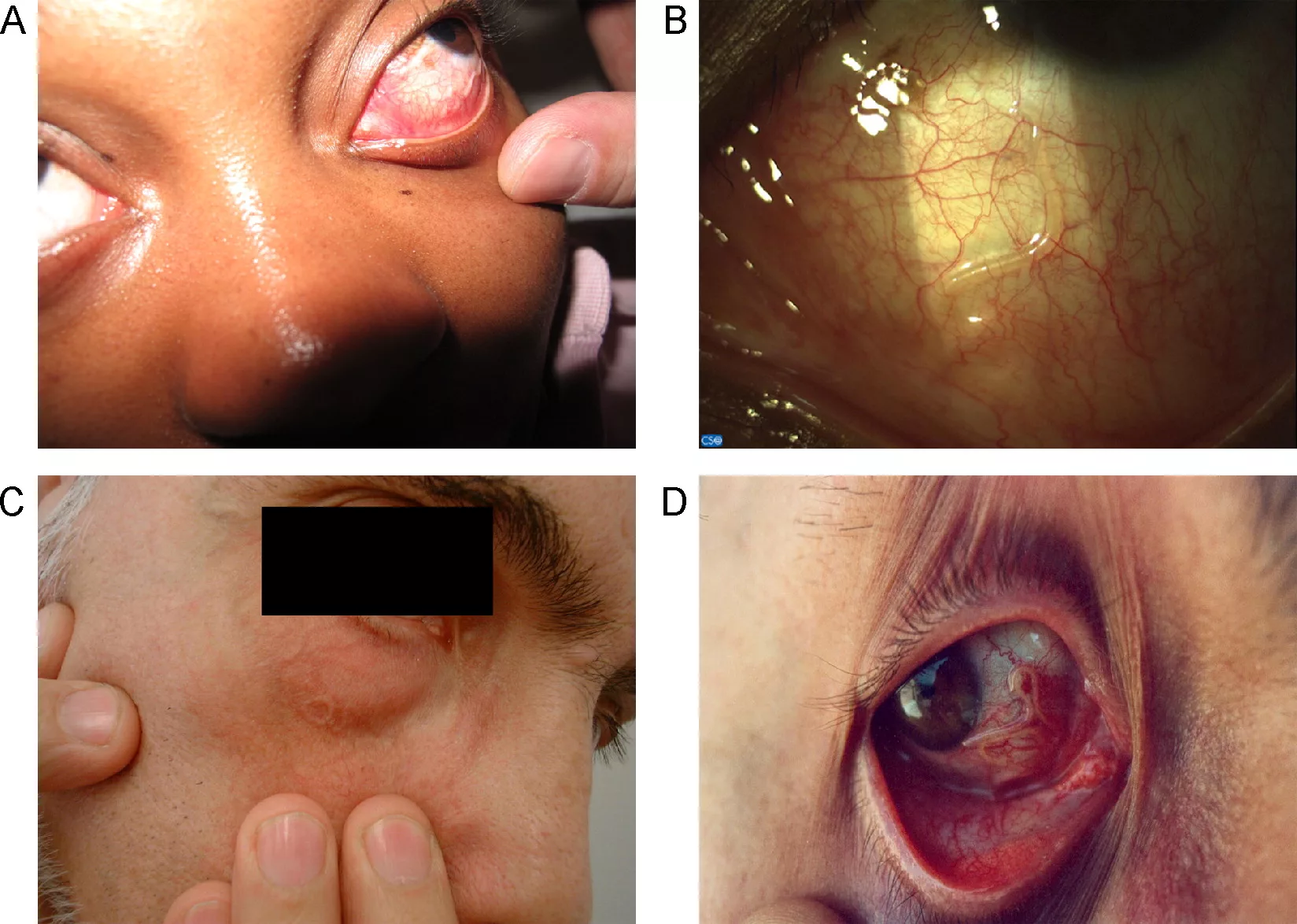
- Angioedema, also known as Calabar edema or Calabar swelling. This symptom is common among the expatriates. Calabar swelling occurs commonly on the arm.
- Hypereosinophilia
- Rash, urticaria, and other allergic skin reactions due to an immune response to microfilariae antigens.
- Fever, arthralgia, and myalgia, resembling systemic inflammatory conditions.
Severe and Rare Complications
In some cases, loiasis can lead to more serious complications, including:9Paul AJ. Loa loa Pathogenesis in Humans. Human Emerging and Re‐emerging Infections: Viral and Parasitic Infections. 2015:441-52.10Buell KG, Whittaker C, Chesnais CB, Jewell PD, Pion SD, Walker M, et al. Atypical Clinical Manifestations of Loiasis and Their Relevance for Endemic Populations. Open Forum Infectious Diseases. 2019: Oxford University Press US.
- Renal complications: Glomerular damage, proteinuria, albuminuria, nephrotic syndrome, and hematuria.
- Cardiovascular involvement: Endomyocardial fibrosis (which can lead to heart failure), thrombotic events, and emboligenic ulcerative endomyocardial fibrosis.
- Neurological issues: Encephalitis, nerve palsies, and ophthalmic complications (partial or complete vision loss).
- Pulmonary involvement: Pulmonary fibrosis and pleural effusion.
- Reproductive system complications: Testicular swelling and possible infertility (though the exact mechanism remains unclear).
- Other systemic effects: Ascites, splenic lesions, acute septic arthritis, and tissue calcification.
Diagnosis of Loiasis
A complete diagnosis of loiasis involves multiple diagnostic approaches.
Clinical Diagnosis:
Healthcare providers can make clinical diagnoses through the Calabar edema. The Calabar edema commonly appears on the face, chest, elbow, and arms. In addition to this, the other most common sign of clinical diagnosis is the ocular passage of the adult worm. The providers can also observe retinal hemorrhage and high microfilaremia in some cases. Neurological, renal, and cardiac complications can also follow the major symptoms. Some additional symptoms that can help establish the diagnosis of loiasis are arthralgia, myalgia, rash, rash, fever, and pruritus. However, symptom analysis alone is insufficient to diagnose the condition.11Paul AJ. Loa loa Pathogenesis in Humans. Human Emerging and Re‐emerging Infections: Viral and Parasitic Infections. 2015:441-52.
Microscopy:
It is the traditional technique to examine blood smears. Microscopy helps to visualize the microfilaria, which are thick and long with a sheath and a terminal nucleus. However, microscopy cannot detect conditions where the microfilaria are not detectable due to low parasitic load. The sampling time also matters as these parasites are more active during the day. It is the gold standard for diagnosis is the identification of microfilariae in a daytime blood smear, as Loa loa exhibits diurnal periodicity, meaning microfilariae circulate in the blood during the day.
Serology:
Serology involves the detection of the specific antigens or antibodies related to loiasis. Elevated levels of IgG4 antibodies are common among people who are even amicrofilaremic.
Enzyme-Linked Immunosorbent Assay (ELISA)
ELISA uses recombinant antigens from L.loa, such as L1-SXP-1, to detect the specific antibodies in the serum of the suspected individuals. The sensitivity of the ELISA for detecting L. loa infection is 15.6% in most cases. The specificity of ELISA is around 99%. This indicates that ELISA can confirm the presence of antibodies but can also miss some cases due to its low sensitivity.12Dieki R, Eyang Assengone E, Nsi Emvo E, Akue J. Profile of loiasis infection through clinical and laboratory diagnostics: the importance of biomarkers. Transactions of The Royal Society of Tropical Medicine and Hygiene. 2023;117(5):349-57.
Luciferase Immunoprecipitation System (LIPS)
This is a newer technique that measures the immune response by detecting specific antibodies. It can achieve around 94% sensitivity and 100% specificity for detecting active infections. LIPS is particularly useful for occult loiasis. This tool diagnoses undetected cases under microscopy and standard serological tools.13Akue JP, Eyang-Assengone E-R, Dieki R. Loa loa infection detection using biomarkers: current perspectives. Research and reports in tropical medicine. 2018:43-8.
Molecular Methods:
The molecular methods used to diagnose loiasis include:
Polymerase Chain Reaction
Healthcare professionals use real-time and nested PCR to diagnose loiasis. These molecular methods have high accuracy for detecting single and mixed infections. PCR is more effective than microscopy, but PCR techniques have disadvantages. These disadvantages include the need of:
- Expensive equipment
- Reliable electrical supply
- Laboratories with incredible infrastructure
- Highly trained staff
- A very long amplification process.14Ta-Tang T-H, Berzosa P, Rubio JM, Romay-Barja M, Ncogo P, Agudo D, et al. Evaluation of LAMP for the diagnosis of Loa loa infection in dried blood spots compared to PCR-based assays and microscopy. Memórias do Instituto Oswaldo Cruz. 2022;116:e210210.
Loop-Mediated Isothermal Amplification (LAMP)
Researchers developed LAMP to overcome the limitations of the PCR. This molecular method is very recent and emerged in 2000. This technology amplifies L. loa DNA from fresh or dried blood spot samples. This technique is advantageous due to its simplicity and lack of complex equipment. It is a field-friendly and cost-effective technique for detecting the disease. The sensitivity of LAMP is id above 90%, while specificity is around 100%. 15Ta-Tang T-H, Berzosa P, Rubio JM, Romay-Barja M, Ncogo P, Agudo D, et al. Evaluation of LAMP for the diagnosis of Loa loa infection in dried blood spots compared to PCR-based assays and microscopy. Memórias do Instituto Oswaldo Cruz. 2022;116:e210210.
Treatment & Management of Loiasis
Currently, healthcare providers use three drugs to treat loiasis.
Albendazole
The physicians typically prescribe albendazole at a dosage of 200- 400 mg two times a day for about 21 days. They usually administer this drug to patients with high worm counts (>8000 mf/mL). It can help reduce microfilariae levels gradually without any severe adverse effects. Albendazole primarily affects the adult worms, lowering the load of worms. It is a safe and effective treatment option for loiasis.16Gobbi F, Bottieau E, Bouchaud O, Buonfrate D, Salvador F, Rojo-Marcos G, et al. Comparison of different drug regimens for the treatment of loiasis—A TropNet retrospective study. PLoS Neglected Tropical Diseases. 2018;12(11):e0006917.
Diethylcarbamazine (DEC)
DEC is the primary treatment option for loiasis. It can target both the larval stages and adult worms. In case of light infection (<8000 mf/mL microfilaria density), the recommended dose of DEC is 6-10 mg/kg for 21 days. It works by enhancing the host’s immune response to the parasites and leading to their destruction. However, in the case of high microfilaria density (>8000 mf/mL), healthcare providers contradict Dec because of the risk of encephalopathy. Moreover, patients can also experience Mazzotti reactions (arthralgia, rash, or fever).17Gobbi F, Bottieau E, Bouchaud O, Buonfrate D, Salvador F, Rojo-Marcos G, et al. Comparison of different drug regimens for the treatment of loiasis—A TropNet retrospective study. PLoS Neglected Tropical Diseases. 2018;12(11):e0006917.
Ivermectin (IVM)
IVM is also an effective treatment option for loiasis, but this drug cannot kill adult worms. Healthcare providers consider IVM as an alternative drug for patients who can not tolerate DEC and when a contradiction of DEC occurs due to high parasitic loads. The recommended dose of IVM is 150 µm/kg. However, IVM can also induce some neurological complications in patients with high parasite loads.18Gobbi F, Bottieau E, Bouchaud O, Buonfrate D, Salvador F, Rojo-Marcos G, et al. Comparison of different drug regimens for the treatment of loiasis—A TropNet retrospective study. PLoS Neglected Tropical Diseases. 2018;12(11):e0006917.
Effective management of loiasis can be through pretreatment evaluation and follow-up care.
- Before starting any treatment, measuring the number of microfilaria circulating in the blood is crucial. This measurement will help determine the most appropriate treatment regimen and assess the adverse complications afterward.
- Regular follow-up appointments are necessary to assess the outcomes of the treatment and related complications.
Prevention of Loiasis
Follow these tips to avoid the infection.
- Use insect repellents on exposed skin and clothes to avoid bites from the deer flies.
- Stay in accommodation with window screens or air conditioning to minimize deerfly exposure.
- Wear full-sleeved shirts and long pants while going outside, mainly during the peak biting hours.
- A clear understanding of the local conditions can help you take adequate precautions.
- Engage yourself with the local health initiative programs to increase awareness about the condition and its transmission.
- Go for regular check-ups if you are a resident of the endemic areas.
- Beware of the symptoms, as early reporting can help in the timely diagnosis and treatment of the disease.
- The healthcare providers recommend DEC when you plan to visit the endemic areas. For long-term travelers to endemic areas, a dose of 300 mg per week can help to avoid loiasis. 19Nutman TB, Miller KD, Mulligan M, Reinhardt GN, Currie BJ, Steel C, et al. Diethylcarbamazine prophylaxis for human loiasis. New England Journal of Medicine. 1988;319(12):752-6. This regimen can reduce the risk of getting a loiasis infection by preventing the development of clinical conditions.
- After returning from a visit to the endemic region of loiasis, you should consult your healthcare provider. They will look for the symptoms of the disease. If there is any suspicion that your exposure to the loa occurs, doctors should perform blood tests to check for the microfilaria.
Final Remarks
Loiasis represents a complex infectious disease with significant health implications in the endemic areas. Traditionally, researchers regard this condition as benign, but later research has proved that it has a link with considerable morbidity and mortality. Continuous research on its epidemiology, pathophysiology, and overall impacts is necessary to improve patient care. Moreover, further research should focus on why loiasis is a neglected tropical disease. By focusing on the condition and creating awareness among people, we can mitigate the spread of this disease. Awareness can lower the burden of loiasis in affected populations.
Refrences
- 1Ramharter M, Butler J, Mombo-Ngoma G, Nordmann T, Davi SD, Manego RZ. The African eye worm: current understanding of the epidemiology, clinical disease, and treatment of loiasis. The Lancet Infectious Diseases. 2024;24(3):e165-e78.
- 2Buell KG, Whittaker C, Chesnais CB, Jewell PD, Pion SD, Walker M, et al., editors. Atypical clinical manifestations of loiasis and their relevance for endemic populations. Open Forum Infectious Diseases; 2019: Oxford University Press US.
- 3Jacobsen KH, Andress BC, Bhagwat EA, Bryant CA, Chandrapu VR, Desmonts CG, et al. A call for loiasis to be added to the WHO list of neglected tropical diseases. The Lancet Infectious Diseases. 2022;22(10):e299-e302.
- 4Burton J. Bogitsh Phd CECP, Thomas N. Oeltmann Phd. Chapter 17 – Blood and Tissue Nematodes. fourth Edition ed2013.
- 5Whittaker C, Walker M, Pion SD, Chesnais CB, Boussinesq M, Basáñez M-G. The population biology and transmission dynamics of Loa loa. Trends in parasitology. 2018;34(4):335-50.
- 6Whittaker C, Walker M, Pion SD, Chesnais CB, Boussinesq M, Basáñez M-G. The population biology and transmission dynamics of Loa loa. Trends in parasitology. 2018;34(4):335-50.
- 7Paul AJ. Loa loa Pathogenesis in Humans. Human Emerging and Re‐emerging Infections: Viral and Parasitic Infections. 2015:441-52.
- 8Paul AJ. Loa loa Pathogenesis in Humans. Human Emerging and Re‐emerging Infections: Viral and Parasitic Infections. 2015:441-52.
- 9Paul AJ. Loa loa Pathogenesis in Humans. Human Emerging and Re‐emerging Infections: Viral and Parasitic Infections. 2015:441-52.
- 10Buell KG, Whittaker C, Chesnais CB, Jewell PD, Pion SD, Walker M, et al. Atypical Clinical Manifestations of Loiasis and Their Relevance for Endemic Populations. Open Forum Infectious Diseases. 2019: Oxford University Press US.
- 11Paul AJ. Loa loa Pathogenesis in Humans. Human Emerging and Re‐emerging Infections: Viral and Parasitic Infections. 2015:441-52.
- 12Dieki R, Eyang Assengone E, Nsi Emvo E, Akue J. Profile of loiasis infection through clinical and laboratory diagnostics: the importance of biomarkers. Transactions of The Royal Society of Tropical Medicine and Hygiene. 2023;117(5):349-57.
- 13Akue JP, Eyang-Assengone E-R, Dieki R. Loa loa infection detection using biomarkers: current perspectives. Research and reports in tropical medicine. 2018:43-8.
- 14Ta-Tang T-H, Berzosa P, Rubio JM, Romay-Barja M, Ncogo P, Agudo D, et al. Evaluation of LAMP for the diagnosis of Loa loa infection in dried blood spots compared to PCR-based assays and microscopy. Memórias do Instituto Oswaldo Cruz. 2022;116:e210210.
- 15Ta-Tang T-H, Berzosa P, Rubio JM, Romay-Barja M, Ncogo P, Agudo D, et al. Evaluation of LAMP for the diagnosis of Loa loa infection in dried blood spots compared to PCR-based assays and microscopy. Memórias do Instituto Oswaldo Cruz. 2022;116:e210210.
- 16Gobbi F, Bottieau E, Bouchaud O, Buonfrate D, Salvador F, Rojo-Marcos G, et al. Comparison of different drug regimens for the treatment of loiasis—A TropNet retrospective study. PLoS Neglected Tropical Diseases. 2018;12(11):e0006917.
- 17Gobbi F, Bottieau E, Bouchaud O, Buonfrate D, Salvador F, Rojo-Marcos G, et al. Comparison of different drug regimens for the treatment of loiasis—A TropNet retrospective study. PLoS Neglected Tropical Diseases. 2018;12(11):e0006917.
- 18Gobbi F, Bottieau E, Bouchaud O, Buonfrate D, Salvador F, Rojo-Marcos G, et al. Comparison of different drug regimens for the treatment of loiasis—A TropNet retrospective study. PLoS Neglected Tropical Diseases. 2018;12(11):e0006917.
- 19Nutman TB, Miller KD, Mulligan M, Reinhardt GN, Currie BJ, Steel C, et al. Diethylcarbamazine prophylaxis for human loiasis. New England Journal of Medicine. 1988;319(12):752-6.