Lymphangioleiomyomatosis, also known as LAM, is a rare, progressive disease that predominantly affects the lungs but can also impact the kidneys and lymphatic system. It is characterized by the abnormal growth of smooth muscle-like cells, known as LAM cells, which invade the lung parenchyma (the functional tissue of the lungs). This invasion leads to the formation of cysts and the gradual destruction of normal lung tissue, resulting in symptoms such as shortness of breath, chest pain, and recurrent pneumothorax (lung collapse).
In the kidneys, LAM is associated with developing angiomyolipomas, benign tumors composed of blood vessels, smooth muscle cells, and fat. These tumors can vary in size and number and may cause complications like bleeding. The disease also affects the lymphatic system, where it can cause lymphatic obstruction, leading to the formation of lymphatic cysts and fluid accumulation in the chest and abdomen.
The reason behind this disease is a specific genetic mutation that affects the normal growth of smooth muscles, and in some cases, you get the disease without any cause. Lymphangioleiomyomatosis mostly affects middle-aged females.1O’Mahony, A. M., Lynn, E., Murphy, D. J., Fabre, A., & McCarthy, C. (2020). Lymphangioleiomyomatosis: a clinical review. Breathe (Sheffield, England), 16(2), 200007. https://doi.org/10.1183/20734735.0007-2020
Causes of Lymphangioleiomyomatosis
You get lymphangioleiomyomatosis in two conditions. Either there is no cause, or it occurs if you are already suffering from tuberous sclerosis complex, and the latter type is the most common. The hormonal changes also cause lymphangioleiomyomatosis as its severity increases with increased estrogen levels in your body. The LAM associated with tuberous sclerosis (TSC-LAM) has an autosomal dominant pattern that involves the mutation of your tumor suppressor genes TSC1 and TSC2 genes, mainly the TSC2. This mutation enhances the activity of the mTOR pathway, leading to increased growth of your smooth muscle cells. The high proliferation of smooth muscle cells becomes the cause of noncancerous tumors in several organs like the lungs and kidneys.2Juvet, S. C., McCormack, F. X., Kwiatkowski, D. J., & Downey, G. P. (2007). Molecular pathogenesis of lymphangioleiomyomatosis: lessons learned from orphans. American journal of respiratory cell and molecular biology, 36(4), 398–408. https://doi.org/10.1165/rcmb.2006-0372TR
Epidemiology of Lymphangioleiomyomatosis
In females, the predominant type of lymphangioleiomyomatosis is TSC-LAM, while the sporadic type is equal in both genders. The average age at which LAM affects females is around 35 to 40, and the sporadic type occurs in almost 3 to 7 cases per million. With the increase in age, the incidence of disease also increases.3Urban T. (2000). Epidémiologie clinique et moléculaire de la lymphangioleiomyomatose et de l’atteinte pulmonaire de la sclérose tubéreuse [Clinical and molecular epidemiology of lymphangioleiomyomatosis and pulmonary pathology in tuberous sclerosis]. Revue des maladies respiratoires, 17(2 Pt 2), 597–603.
Types of Lymphangioleiomyomatosis
The two types of lymphangioleiomyomatosis are:
1. TSC-LAM
This is the most common type that occurs in association with tuberous sclerosis, which is also a genetic disease where you get epilepsy, mental disability, and tumors in multiple parts of the body. You can also transmit it to the next generation in an autosomal dominant pattern.
2. S-LAM
The sporadic type occurs randomly, mostly in females of premenopausal age, and it doesn’t transmit from one generation to another.
Symptoms of Lymphangioleiomyomatosis
Symptoms of lymphangioleiomyomatosis depend upon the organ involved. These are:
Lungs:
Lungs are the main organ involved in LAM. You present with the following symptoms related to the respiratory system:
Dyspnea (Shortness of Breath)
Dyspnea is the main complaint you have in LAM disease. In the initial stages, your physician might diagnose this as a symptom of chronic obstructive progressive disease (COPD).
Fatigue
Fatigue presents in the initial stages of LAM in almost half of the patients.
Pleural Effusion
It is a collection of water in the layers of your lungs, either due to an infection, malignancy, or heart disease. Many patients with lymphangioleiomyomatosis suffer from this condition, and they also feel chest pain due to effusion.
Pneumothorax
The presence of air in your pleural cavity may cause your lung to collapse due to its pressure.
Hemoptysis
Cough with mucus production is observed in patients with lymphangioleiomyomatosis, and sometimes, hemoptysis (the presence of blood in the cough) occurs, which is considered one of the common symptoms of lymphangioleiomyomatosis.
Wheezing
Wheezing (a whistling sound in your chest) might be present due to severe shortness of breath, which your physician diagnoses during chest auscultation.
Lymphatics:
When lymphangioleiomyomatosis involves the lymphatics, the following conditions may appear in your body:
Thoracic Duct Dilatation
The thoracic duct returns the lymph in your systemic circulation. When LAM affects the lymphatics, lymph cannot flow properly, causing the thoracic duct to dilate.
Chylothorax
Chylothorax occurs when lymph, a fluid rich in fats, proteins, and white blood cells, leaks into the thoracic cavity, the space surrounding the lungs. This leakage can result from lymphatic obstruction, a common complication in lymphangioleiomyomatosis (LAM). Symptoms of chylothorax include shortness of breath and cough due to fluid accumulation in the pleural space around the lungs. In LAM, chylothorax is a significant clinical presentation that requires prompt management.
Chylous Ascites
An accumulation of triglyceride-rich white-colored liquid in your abdominal cavity occurs due to lymphatic injury and obstruction.
Kidneys:
Tumors develop in your kidneys, like angiomyolipomas (benign tumors), when affected by lymphangioleiomyomatosis, mostly in both kidneys.
Brain:
Involvement of the brain in lymphangioleiomyomatosis presents as follows:
Epilepsy
Another name for epilepsy is seizures resulting from abnormal electrical impulses in your brain. Epilepsy also has a genetic background, just like lymphangioleiomyomatosis, causing an increased chance of its occurrence. Epilepsy also affects cognitive abilities in a way that you lose your attention, short-term memory, and mental abilities.
Cortical Hamartomas
These are a type of brain tumor resulting from the abnormal growth of your normal brain cells, and their typical feature is seizures. In the neurological manifestation of LAM, hamartomas occur too often.
Astrocytoma
Your brain and spinal cord have specific cells called astrocytes, and when an abnormal growth occurs in these cells, it leads to the formation of a tumor called astrocytoma. In TSC-LAM, astrocytoma presents more frequently due to its association with tuberous sclerosis.
Skin:
When the skin is involved in lymphangioleiomyomatosis, you get hypopigmented macules due to decreased production of melanin in the skin, plaques on the forehead, or fibroadenomas on your face. Defects in the nails, like grooves, are also seen in patients with lymphangioleiomyomatosis.4Zhang, H., Hu, Z., Wang, S., Wu, K., Yang, Q., & Song, X. (2022). Clinical features and outcomes of male patients with lymphangioleiomyomatosis: A review. Medicine, 101(52), e32492. https://doi.org/10.1097/MD.0000000000032492
Risk Factors of Lymphangioleiomyomatosis
Lymphangioleiomyomatosis does not have specific risk factors as it presents either randomly in life or due to a genetic defect. However, females in their premenopausal age (35-40 years) are more at risk of getting LAM. Moreover, females who take estrogen pills get lymphangioleiomyomatosis more often than those who don’t take the pills.
Diagnosis of Lymphangioleiomyomatosis
The first step in diagnosing lymphangioleiomyomatosis is taking a complete history and performing a physical examination.
History & Physical Examination:
To take a comprehensive history, your physician inquires about your symptoms, such as shortness of breath, cough, and fatigue, and also symptoms related to the brain, like memory loss or seizures. During the physical examination, the doctor auscultates your chest with a stethoscope to observe whether you have wheezing. Examination of the face, skin, and nails is crucial as the disease presents with abnormalities like plaques and fibroadenomas.
Laboratory Tests:
Lymphangioleiomyomatosis does not affect your CBC or has no specific biomarkers to detect. However, you may check the following important things to diagnose it:
VEGF-D Levels
Studies show that if you have lymphangioleiomyomatosis, the level of vascular endothelial growth factor D increases in your body by as much as 800 pg/mL. So, it is essential to get your VEGF-D levels checked.
Triglyceride Level in Pleural Effusion
If you have pleural effusion and lymphangioleiomyomatosis, your physician may perform thoracentesis (taking a fluid sample from your pleural cavity) and get it checked in the laboratory. This effusion is mainly chylous due to the obstruction of your lymphatics, and you also observe an increased level of triglycerides in the fluid.
Pulmonary Function Tests:
Pulmonary function tests are crucial as your lungs are the primary organs affected by lymphangioleiomyomatosis. The spirometry exhibits a decline in FEV1/FVC ratio and DLCO (decreasing capacity of your lungs for CO). You may undergo a six-minute walk test to determine the severity of hypoxia (reduced oxygen) in the body.
Radiological Tests:
Imaging is crucial in diagnosing lymphangioleiomyomatosis because it causes pneumothorax, cysts, kidney tumors, and many other presentations easily detected by imaging studies.
Chest X-Ray
Chest X-rays are important in diagnosing various diseases, but they don’t play a significant role in LAM. However, if you suspect pneumothorax or pleural effusion, it is better to get a chest X-ray, as it is considered a good investigation to diagnose these diseases.
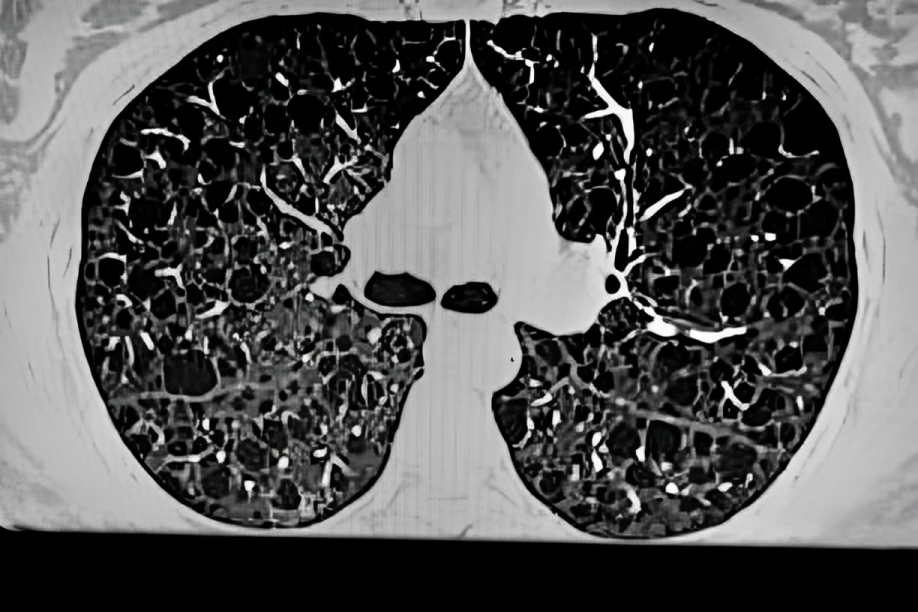
HRCT
A High-resolution CT scan clearly shows the presence of thin-walled cysts in both lungs, making it a recommended investigation for diagnosing lymphangioleiomyomatosis. HRCT also helps diagnose other disease features like pleural thickening, inflated lungs, a ground-glass appearance in the lungs, and pulmonary hemorrhage.
Abdominal CT Scan or Ultrasound
Lymphangioleiomyomatosis is known to form cysts and tumors in the kidneys, like angiomyolipomas, which is why your doctor may suggest you have an abdominal ultrasound and CT scan to rule out kidney involvement.
CT or MRI of the Brain
A CT or MRI of the brain detects the changes in your brain due to lymphangioleiomyomatoses, such as the presence of any tumor, like meningioma or astrocytoma.
Bone Densitometry
Bone densitometry, or DEXA scan, measures bone mineral density in patients with lymphangioleiomyomatosis (LAM). While LAM itself does not directly affect the bones, patients are at an increased risk of developing osteoporosis due to factors like long-term corticosteroid use and reduced physical activity. Bone densitometry can help detect osteoporosis, indicating weakened bones that are more prone to fractures
Lung Biopsy:
The best investigation that your doctor may suggest to diagnose lymphangioleiomyomatosis is a lung biopsy. There are several methods by which a lung biopsy is done, and the choice of procedure is made by the patient’s will, underlying diseases, and doctor’s experience. These approaches are video-assisted thoracoscopy, surgical wedge resection, and trans-bronchial lung biopsy; the first is not recommended due to unsatisfactory results. A microscope is used to observe the piece of lung taken in the biopsy. It clearly shows the abnormal smooth muscle cells, which are the basic cause of lymphangioleiomyomatosis.5Xu, W., Cui, H., Liu, H., Feng, R., Tian, X., Yang, Y., & Xu, K. F. (2021). The value of transbronchial lung biopsy in the diagnosis of lymphangioleiomyomatosis. BMC pulmonary medicine, 21(1), 146. https://doi.org/10.1186/s12890-021-01518-2

Histopathology & Biomarkers:
After a piece of the lung is taken through a biopsy, it undergoes histopathological tests in the laboratory using H and E stains. These stains clearly show the proliferation of smooth muscle cells and your lungs’ cystic spaces. The stains also show specific biomarkers on which diagnosis of LAM is made. These are HMB-45, actin, myosin, estrogen, or progesterone.6Gupta, N., Finlay, G. A., Kotloff, R. M., Strange, C., Wilson, K. C., Young, L. R., Taveira-DaSilva, A. M., Johnson, S. R., Cottin, V., Sahn, S. A., Ryu, J. H., Seyama, K., Inoue, Y., Downey, G. P., Han, M. K., Colby, T. V., Wikenheiser-Brokamp, K. A., Meyer, C. A., Smith, K., Moss, J., … ATS Assembly on Clinical Problems (2017). Lymphangioleiomyomatosis Diagnosis and Management: High-Resolution Chest Computed Tomography, Transbronchial Lung Biopsy, and Pleural Disease Management. An Official American Thoracic Society/Japanese Respiratory Society Clinical Practice Guideline. American journal of respiratory and critical care medicine, 196(10), 1337–1348. https://doi.org/10.1164/rccm.201709-1965ST
Treatment & Management of Lymphangioleiomyomatosis
In the initial stage of lymphangioleiomyomatosis, you present to a doctor complaining of shortness of breath and airway obstruction. Your doctor may diagnose it as a respiratory disease like asthma or COPD.
The treatment for the respiratory symptoms is:
Bronchodilators:
Bronchodilators are effective when the spirometry results show that the disease is reversible. To relieve your symptoms, your doctor may prescribe beta-agonists like albuterol and anticholinergics like ipratropium.
In addition to the symptomatic treatment, there are other modalities to treat lymphangioleiomyomatosis. These are:
Medical Approach:
The basic mechanism of lymphangioleiomyomatosis is the overactivation of mTOR pathways, eventually leading to abnormal growth of your smooth muscle cells. Based on this mechanism, a medicine named sirolimus is formulated to inhibit the activity of the mTOR pathway and is considered most effective against LAM. Sirolimus not only improves your respiratory symptoms but also helps reduce VEGF-D levels and the manifestation of chylous effusion in your body.
Your doctor recommends a daily dose of 1mg of sirolimus, which should be taken orally. You might get an allergy to sirolimus and other adverse effects like nausea, vomiting, dyspepsia, acne, infection, or anemia. In the case of these symptoms, your physician recommends an alternative called everolimus, especially in TSC-LAM.
Surgical Approach:
Lung transplantation is recommended for patients who are not improving with the medical treatment. However, lymphangioleiomyomatosis can still reoccur even after lung transplantation in some patients.
Pleurodesis:
If you have severe pleural effusion, pneumothorax, and lymphangioleiomyomatosis, your doctor might suggest pleurodesis. In this procedure, the surgeon puts a drug in your pleural cavity that helps the lung to stick to the chest wall. In that way, you can prevent reoccurrence of effusion or pneumothorax.7Taveira-DaSilva, A. M., & Moss, J. (2014). Management of lymphangioleiomyomatosis. F1000prime reports 6, 116. https://doi.org/10.12703/P6-116
Differential Diagnoses of Lymphangioleiomyomatosis
Symptoms of lymphangioleiomyomatosis resemble the symptoms of some other diseases. That is why it is crucial to have proper examinations and investigations to rule out other morbidities. Important differentials are:
Asthma:
The initial symptoms of lymphangioleiomyomatosis resemble a lot of asthma, like severe shortness of breath, productive cough, and occasionally chest pain. It is crucial to rule out asthma by performing pulmonary function tests and chest X-rays.
Amyloidosis:
Amyloidosis mainly affects your kidneys by forming abnormal proteins. Just like lymphangioleiomyomatosis, it also causes kidney failure and shortness of breath. A biopsy is the main investigation to differentiate these two conditions.8Xu, K. F., & Lo, B. H. (2014). Lymphangioleiomyomatosis: differential diagnosis and optimal management. Therapeutics and clinical risk management, 10, 691–700. https://doi.org/10.2147/TCRM.S50784
Prognosis of Lymphangioleiomyomatosis
The recurrence of lymphangioleiomyomatosis, even if you have lung transplantation, makes it a disease with a poor prognosis. Life span also decreases in patients with this disease. Moreover, you can still get pneumothorax and effusion even after having pleurodesis. 9Zak, S., Mokhallati, N., Su, W., McCormack, F. X., Franz, D. N., Mays, M., Krueger, D. A., Szczesniak, R. D., & Gupta, N. (2019). Lymphangioleiomyomatosis Mortality in Patients with Tuberous Sclerosis Complex. Annals of the American Thoracic Society, 16(4), 509–512. https://doi.org/10.1513/AnnalsATS.201807-471RL
Precautions of Lymphangioleiomyomatosis
Although lymphangioleiomyomatosis has a very poor prognosis, you should still take some precautions to limit its progression. Pulmonary cysts may rupture if you travel by air, so it is better to avoid them. The use of estrogen aggravates symptoms of disease, so you should avoid taking estrogen contraceptive pills. It would help if you get vaccinated against influenza and pneumococcal infections.
Conclusion
To conclude, lymphangioleiomyomatosis is a smooth muscle cell defect that causes it to grow abnormally. The reason behind this defect is whether it is a genetic mutation or may occur randomly in your life. LAM largely affects females between 35 to 40 years of age, mainly affecting their lungs. Kidney tumors and lymphatic obstruction are also seen in patients with lymphangioleiomyomatosis. Use sirolimus to treat this disease, and you can also opt for lung transplantation if medical treatment is not helping.
Refrences
- 1O’Mahony, A. M., Lynn, E., Murphy, D. J., Fabre, A., & McCarthy, C. (2020). Lymphangioleiomyomatosis: a clinical review. Breathe (Sheffield, England), 16(2), 200007. https://doi.org/10.1183/20734735.0007-2020
- 2Juvet, S. C., McCormack, F. X., Kwiatkowski, D. J., & Downey, G. P. (2007). Molecular pathogenesis of lymphangioleiomyomatosis: lessons learned from orphans. American journal of respiratory cell and molecular biology, 36(4), 398–408. https://doi.org/10.1165/rcmb.2006-0372TR
- 3Urban T. (2000). Epidémiologie clinique et moléculaire de la lymphangioleiomyomatose et de l’atteinte pulmonaire de la sclérose tubéreuse [Clinical and molecular epidemiology of lymphangioleiomyomatosis and pulmonary pathology in tuberous sclerosis]. Revue des maladies respiratoires, 17(2 Pt 2), 597–603.
- 4Zhang, H., Hu, Z., Wang, S., Wu, K., Yang, Q., & Song, X. (2022). Clinical features and outcomes of male patients with lymphangioleiomyomatosis: A review. Medicine, 101(52), e32492. https://doi.org/10.1097/MD.0000000000032492
- 5Xu, W., Cui, H., Liu, H., Feng, R., Tian, X., Yang, Y., & Xu, K. F. (2021). The value of transbronchial lung biopsy in the diagnosis of lymphangioleiomyomatosis. BMC pulmonary medicine, 21(1), 146. https://doi.org/10.1186/s12890-021-01518-2
- 6Gupta, N., Finlay, G. A., Kotloff, R. M., Strange, C., Wilson, K. C., Young, L. R., Taveira-DaSilva, A. M., Johnson, S. R., Cottin, V., Sahn, S. A., Ryu, J. H., Seyama, K., Inoue, Y., Downey, G. P., Han, M. K., Colby, T. V., Wikenheiser-Brokamp, K. A., Meyer, C. A., Smith, K., Moss, J., … ATS Assembly on Clinical Problems (2017). Lymphangioleiomyomatosis Diagnosis and Management: High-Resolution Chest Computed Tomography, Transbronchial Lung Biopsy, and Pleural Disease Management. An Official American Thoracic Society/Japanese Respiratory Society Clinical Practice Guideline. American journal of respiratory and critical care medicine, 196(10), 1337–1348. https://doi.org/10.1164/rccm.201709-1965ST
- 7Taveira-DaSilva, A. M., & Moss, J. (2014). Management of lymphangioleiomyomatosis. F1000prime reports 6, 116. https://doi.org/10.12703/P6-116
- 8Xu, K. F., & Lo, B. H. (2014). Lymphangioleiomyomatosis: differential diagnosis and optimal management. Therapeutics and clinical risk management, 10, 691–700. https://doi.org/10.2147/TCRM.S50784
- 9Zak, S., Mokhallati, N., Su, W., McCormack, F. X., Franz, D. N., Mays, M., Krueger, D. A., Szczesniak, R. D., & Gupta, N. (2019). Lymphangioleiomyomatosis Mortality in Patients with Tuberous Sclerosis Complex. Annals of the American Thoracic Society, 16(4), 509–512. https://doi.org/10.1513/AnnalsATS.201807-471RL