Retroperitoneal fibrosis (RPF) is a rare disorder characterized by the development of fibrous tissue and chronic inflammation within the retroperitoneum. The retroperitoneum is a space between your abdominal cavity and the back muscles (the posterior parietal peritoneum and the posterior abdominal wall). This fibrotic tissue can encase and compress retroperitoneal structures, particularly the ureters, leading to obstruction, as well as exert pressure on major vessels like the aorta, inferior vena cava, and superior mesenteric vessels.
Symptoms of this condition depend on the organ under pressure due to fibrosis. Most cases of RF are idiopathic, which means they develop spontaneously and without any apparent cause. However, some patients may develop retroperitoneal fibrosis due to any drug or if they have an already existing immune-related disorder.1Engelsgjerd, J. S., Leslie, S. W., & LaGrange, C. A. (2024). Retroperitoneal Fibrosis. In StatPearls. StatPearls Publishing.
Causes of Retroperitoneal Fibrosis
Important causes of retroperitoneal fibrosis are:2 Runowska, M., Majewski, D., & Puszczewicz, M. (2016). Retroperitoneal fibrosis – the state-of-the-art. Reumatologia, 54(5), 256–263. https://doi.org/10.5114/reum.2016.63667
Idiopathic:
Retroperitoneal fibrosis mainly develops idiopathically. More than 70% of cases develop without a known cause, mainly due to Immunoglobulin-4 (IgG4). Almost 35-60% of patients with idiopathic RF have an IgG-4-related disease, making it the most crucial cause behind retroperitoneal fibrosis.3 Vaglio, A., & Maritati, F. (2016). Idiopathic Retroperitoneal Fibrosis. Journal of the American Society of Nephrology : JASN, 27(7), 1880–1889. https://doi.org/10.1681/ASN.2015101110
Drugs:
Some drugs that play a secondary role in developing RF include:
- Methysergide (nearly 12% of cases of retroperitoneal fibrosis develop due to this drug)
- Methyldopa
- Bromocriptine
- Beta-blockers
- Analgesics
- Hydralazine
- Tumor necrosis factor (TNF) inhibitors, such as etanercept and infliximab
Malignancies:
Retroperitoneal fibrosis can also develop in patients with certain malignancies, and these cancers account for almost 8% of the cases. Lymphoma, such as Hodgkin’s and non-Hodgkin lymphoma, is the leading cause of RF. In addition to lymphomas, other malignancies that play a significant role in causing retroperitoneal fibrosis include sarcomas, colorectal, breast, and prostate cancer.
Infections:
Chronic infections such as tuberculosis, histoplasmosis, and actinomycosis may initiate RF. Radiation therapy for abdominal or pelvic cancers—like testicular seminoma, colon, or pancreatic cancer—can also lead to fibrosis in the retroperitoneum. Additionally, surgical interventions such as lymphadenectomy may trigger fibrotic changes if complications occur.
Asbestos:
Asbestos exposure can also cause retroperitoneal fibrosis, especially if the exposure continues for an extended period. People who work with building and insulation materials are more exposed to asbestos and, hence, can easily acquire retroperitoneal fibrosis. There is at least an 8-12-fold increased risk of retroperitoneal fibrosis in people who have asbestos exposure, along with a strong smoking history.
Epidemiology of Retroperitoneal Fibrosis
Retroperitoneal fibrosis is an uncommon condition, with an annual incidence of approximately 1.3 to 1.4 per 100,000 people. It most frequently affects middle-aged adults, particularly between 40 and 60 years old, but can also present in older individuals and, rarely, children. Males are affected more often than females, with a male-to-female ratio of 2:1 to 3:1.
Pathophysiology behind Retroperitoneal Fibrosis
The exact mechanism behind idiopathic RF is not fully understood but is thought to involve an autoimmune or immune-mediated inflammatory process. It may co-occur with chronic inflammatory diseases such as chronic pancreatitis, which can initiate a periaortic inflammatory response. In this case, the lab’s findings will show increased acute-phase reactants and autoantibodies in the body. The inflammatory response in the body in patients with retroperitoneal fibrosis is a combined action of specific cytokines and chemokines, most commonly TGF-β, CXCL11, and CXCL12. They initiate the process of fibrosis along with the actions of some interleukins, such as IL-4, IL-6, IL-11, and IL-12. Retroperitoneal fibrosis that develops due to drug interaction has no specific pathophysiology.
IgG-4-mediated disease that causes retroperitoneal fibrosis in the body has an incidence of almost 27%, and patients have increased levels of plasma cells that secrete IgG4 in their bodies. These IgG-4-mediated diseases can also give rise to isolated retroperitoneal fibrosis. However, in most cases, it clinically presents as the swelling of affected organs, most commonly the kidneys, pancreas, retroperitoneum, and salivary glands.
This form of RPF typically affects older men and may coexist with renal involvement, nephritis, or renal masses. Lab tests in IgG4-RPF patients often show elevated eosinophils, C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), creatinine, and low complement levels.
Diagnosing IgG-4-mediated retroperitoneal fibrosis is difficult, as it has complex presentations involving many organs. It also has a higher risk of causing pancreatic cancer and lymphoma.
Symptoms of Retroperitoneal Fibrosis
More than 90% of patients with this condition present with the initial symptoms of dull and vague pain in their abdomen, back, inguinal, or flank areas. In some cases, retroperitoneal fibrosis puts pressure on the testis, resulting in testicular pain in more than 50% of patients. The pain in RF can radiate to the inguinal area but cannot be adequately localised. There is often a worsening of pain at night, and changing body position does not affect the pain of retroperitoneal fibrosis.4 Renganathan, G., Thangarasu, S., Pathak, N., Kunam, V. K., & Afzal, Z. (2024). The Enigma of Retroperitoneal Fibrosis: Clinical Implications and Diagnostic and Therapeutic Challenges. Cureus, 16(11), e74499. https://doi.org/10.7759/cureus.74499
The pressure of RF severely affects the ureters, leading to hydronephrosis (swelling of the renal pelvis) and obstructive uropathy.
The continuous compression of retroperitoneal fibrosis affects the inferior vena cava, and its obstruction causes serious complications like deep venous thrombosis and oedema of the legs. Severe cases of RF affect the patients’ mesenteric arteries, causing disruptions in the blood supply. Patients present with leg claudication (pain while walking) in the case of obstructive mesenteric arteries.
The advanced cases of RF present as ascites (fluid in the abdomen), obstruction of the bowel, or compression of the spinal cord.5Jadhav, K. K., Kumar, V., Punatar, C. B., Joshi, V. S., & Sagade, S. N. (2017). Retroperitoneal fibrosis-clinical presentation and outcome analysis from a urological perspective. Investigative and clinical urology, 58(5), 371–377. https://doi.org/10.4111/icu.2017.58.5.371 Some rare symptoms are:
- Anuria (no urine production) and oliguria (decrease in urine production)
- Anorexia (loss of hunger)
- Weight loss
- Malaise
- Unexplained fever
- Nausea and vomiting
- Hydrocele (fluid in the space around the testicles)
- Varicocele (enlarged venous plexus around the testis)
How to diagnose Retroperitoneal Fibrosis?
The accurate diagnosis requires the following essential steps:6Tanaka, T., & Masumori, N. (2020). Current approach to diagnosis and management of retroperitoneal fibrosis. International journal of urology : official journal of the Japanese Urological Association, 27(5), 387–394. https://doi.org/10.1111/iju.14218
History:
The diagnosis starts with taking a history and then doing a proper physical examination. Retroperitoneal fibrosis is diagnosed incidentally in patients who present with vague abdominal pain, oedema, or urinary tract obstruction.
To diagnose RF, it is essential to take a history of pain, including its nature, duration, and frequency. Moreover, taking a history of kidney-related issues like decreased urine output, pai,n and swelling in the flank area is also essential.
Physical Examination:
During physical examination of a suspected patient, it is essential to examine any tenderness in the abdomen, especially at the costovertebral angle. Checking the blood pressure is vital, as almost 57% of patients with retroperitoneal fibrosis present with hypertension. Physical examination of the testis may reveal the presence of a hydrocele or tenderness. Looking for signs of oedema, thrombophlebitis, and deep vein thrombosis also helps diagnose RF.
Laboratory Studies:
Laboratory studies are not specific and aren’t enough to diagnose the disease, as results vary from person to person. However, you may find the following changes in the laboratory tests:
- Increase in the level of alkaline phosphatase. It is a valuable marker for discovering the activity of the disease.
- In case of urinary obstruction, the level of urea and creatinine increases. Kidney failure in this condition may lead to normocytic normochromic anemia.
- In idiopathic RF, the antinuclear antibody (ANA) level is high in almost 60% of patients. It also causes an increase in eosinophils. Moreover, doctors also advise thyroid function tests in idiopathic RF, as it may present with autoimmune thyroiditis.
- Levels of ESR and CRP might be raised due to inflammation in this condition, and these levels also help to see the treatment results.
- Some physicians recommend checking the levels of autoantibodies in your body, like antithyroid microsomal, anti-thyroglobulin, or anti-smooth muscle antibodies, if there is a suspicion of immune-mediated RF.
- Serum protein electrophoresis is helpful in rare cases to rule out plasma cell dyscrasias.
Renal Ultrasound:
It is usually the first-line imaging in suspected cases. It may show hydronephrosis due to ureteral obstruction. An irregular, ill-defined periaortic mass may also be seen in the retroperitoneum.7Chen, S. J., Qin, L., Xie, Y. J., Zhu, J. P., Zhang, Q., & Chen, M. (2017). Ultrasonography for Preoperative Diagnosis of Retroperitoneal Fibrosis. Ultrasound quarterly, 33(2), 162–166. https://doi.org/10.1097/RUQ.0000000000000293
Contrast-Enhanced CT Scan & MRI:
A contrast-enhanced CT scan helps determine how much retroperitoneal fibrosis has spread. It also detects the presence of lymphadenopathy and any underlying tumour. Intravenous contrast is used to visualise the underlying pathology, and the resulting enhancement changes with increasing age.
Mass present in the retroperitoneum affects multiple organs like the aorta and inferior vena cava. Moreover, a CT scan shows the presence of hydronephrosis and medial deviation of the ureters. In the case of IgG-4-mediated retroperitoneal fibrosis, a contrast-enhanced CT scan reveals the presence of many small nodular lesions in your renal pelvis.
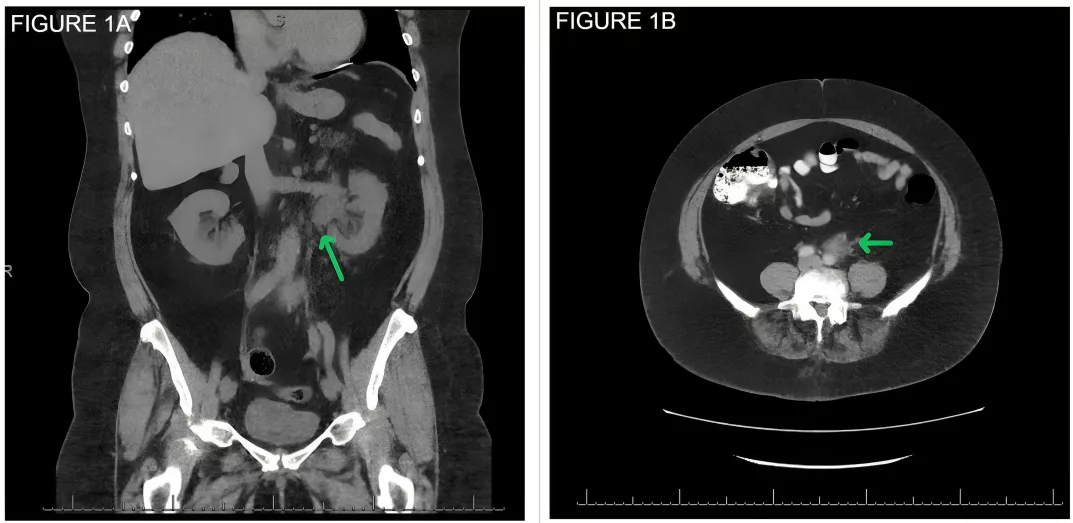
Hydronephrosis on the left due to a retroperitoneal mass. Note the left-
sided hydronephrosis and proximal hydroureter, and (B) A 2.8 x 1.7 cm
retroperitoneal soft tissue mass along the anterior margin of the aorta
and abutting the mid-segment of the left ureter. Image courtesy: Bilateral hydronephrosis from retroperitoneal fibrosis.ResearchGate, 2020. Available at: Researchgate under license CC BY 3.0
The reliability of an MRI is the same as that of a CT scan in detecting retroperitoneal fibrosis. However, MRI is more helpful in finding the disease activity and progression. When retroperitoneal fibrosis presents with a tumour, an MRI shows anterior elevation of the aorta, destruction of the bone, a large mass, and deviation of the ureters.8Mulligan, S. A., Holley, H. C., Koehler, R. E., Koslin, D. B., Rubin, E., Berland, L. L., & Kenney, P. J. (1989). CT and MR imaging in the evaluation of retroperitoneal fibrosis. Journal of Computer Assisted Tomography, 13(2), 277–281. https://doi.org/10.1097/00004728-198903000-00018
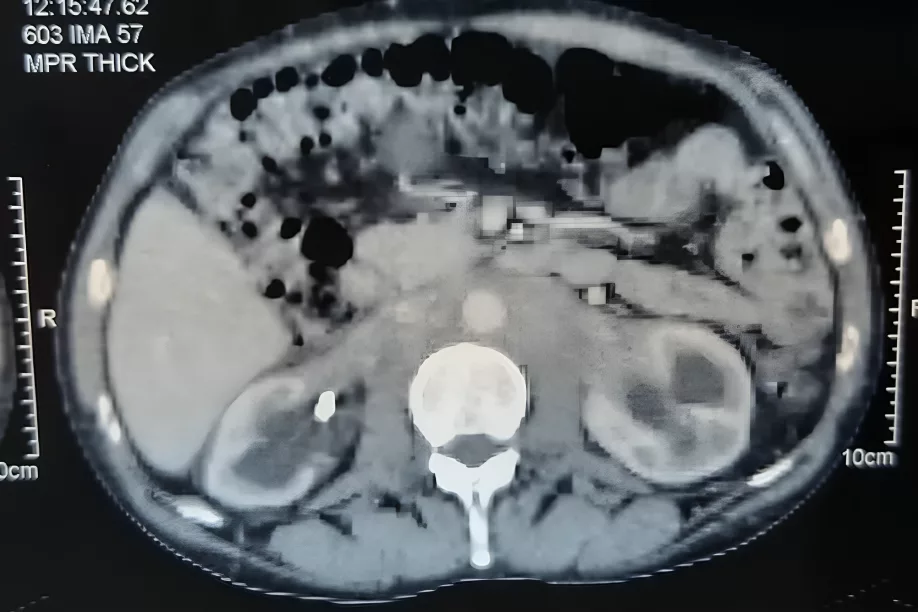
retroperitoneal fibrosis with a perivascular fibrous sheath and bilateral
hydro nephrosis. Image courtesy : Retroperitoneal fibrosis: Beware of lymphoma. ResearchGate 2020. Available at: Researchgate under license CC BY 3.0
Positron Emission Tomography:
A PET scan is not an investigation of choice for retroperitoneal fibrosis. However, if there is a need to discover disease progression, PET scans are more reliable than CT and MRI scans. It also helps to identify the spread of the disease to other sites.
Before taking a biopsy, doctors use a PET scan to find the exact location where the disease activity is higher.9Wang, Y., Guan, Z., Gao, D., Luo, G., Li, K., Zhao, Y., Wang, X., Zhang, J., Jin, J., Zhao, Z., Yang, C., Zhang, J., Zhu, J., & Huang, F. (2018). The value of 18F-FDG PET/CT in the distinction between retroperitoneal fibrosis and its malignant mimics. Seminars in arthritis and rheumatism, 47(4), 593–600. https://doi.org/10.1016/j.semarthrit.2017.07.011
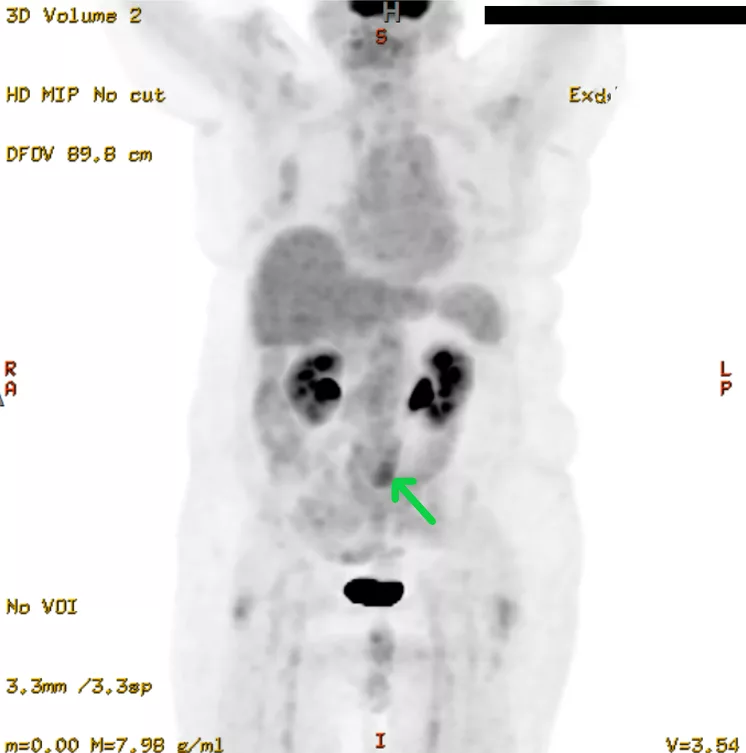
retroperitoneal mass. Image courtesy: Bilateral hydronephrosis from retroperitoneal fibrosis.ResearchGate, 2020. Available at: Researchgate under license CC BY 3.0
Lymphangiography:
Doctors usually do not recommend lymphangiography for the diagnosis of retroperitoneal fibrosis. However, if a CT scan and MRI fail to diagnose the disease and there is still a suspicion of this condition, lymphangiography is considered. This disease can affect your lymphatics even before affecting the ureters, as lymphatics have a delicate structure. The results of lymphangiography show blocked lymphatics at the level of L3/L4 and a defective system of lymph drainage, mostly in iliac and para-aortic lymph nodes.
Tissue biopsy:
Tissue Biopsy is not routinely required, but should be considered if:
- Malignancy is suspected
- Atypical imaging features are present
- There’s no response to glucocorticoid therapy
- There’s significant lymphadenopathy or aortic displacement
A CT-guided core needle biopsy is often preferred, focusing on the most active or thickened areas. Biopsy may show dense fibrosis with inflammatory infiltrates, and IgG4 plasma cell staining in IgG4-related disease.
Treatment of Retroperitoneal Fibrosis
Treatment depends on the initial clinical presentation of the patients. It comprises initial acute management, medical treatment with drugs, and survival intervention.
Initial Acute Management:
In cases of ureteral obstruction, hydronephrosis, or uremia, immediate decompression of the urinary tract is required using percutaneous nephrostomy or double J (DJ) stents.
DJ stents are easier to insert and remove and are often sufficient if the patient responds to steroid therapy. They also allow retrograde pyelography, which helps visualise the internal structure of the ureters.
Patients often develop post-obstructive diuresis (urine output >200 mL/hr) once the obstruction is relieved, which can lead to dehydration and electrolyte imbalance. Initial management includes oral fluids, but IV hydration with half-normal saline may be required if oral intake is inadequate.
In patients with symptoms of vascular obstruction, immediate intervention with angioplasty or stenting may be necessary.
Once the patient is stabilised, it is essential to evaluate the underlying cause before initiating long-term treatment.
Medical Treatment:
The most effective primary medical treatment for this disease is glucocorticoid therapy, which resolves almost 80% of cases. The recommended period for glucocorticoid therapy is 4 weeks. Steroid therapy helps in reducing pain and improving diuresis, and also causes a decrease in ESR levels. Renal function can improve within 2 weeks of taking steroid therapy.10 Scheel, P. J., Jr, Sozio, S. M., & Feeley, N. (2012). Medical management of retroperitoneal fibrosis. Transactions of the American Clinical and Climatological Association, 123, 283–291.
Physicians recommend oral prednisolone at a dose of 1mg/kg/day ( maximum dose of 80mg ) for at least 4 weeks. If there is an improvement in symptoms with this dose, the steroid therapy is gradually reduced to at least 10 mg/d. Some doctors prescribe prednisolone at a dose of 60 mg.
Despite the many advantages of using steroids, they can also cause many side effects. Therefore, patients should take calcium, vitamin D supplements, and steroids to avoid osteoporosis. Some other harmful effects of glucocorticoid therapy are:
- Diabetes mellitus
- Cushing syndrome
- Cataracts and glaucoma
- Hypertension
- Obesity
- Peptic ulcer disease
In case of failure of glucocorticoid therapy, it is better to reevaluate the cause of this condition either by CT scan or tissue biopsy. Some other drugs recommended by doctors to use along with steroids are:
Immunosuppressants
These include azathioprine, methotrexate, colchicine, and mycophenolate mofetil. These drugs are typically used in different conditions, but they all help reduce the severity of idiopathic retroperitoneal fibrosis.
Additional Agents
- Rituximab, a monoclonal antibody, helps to resolve the refractory cases of this fibrosis.
- Tamoxifen, an antiestrogen, also has a fair success rate. Other agents are cyclophosphamide, progesterone, and cyclosporine.
Surgical Treatment:
Surgical treatment is done in patients with ureteral obstruction and hydronephrosis. It involves removing the ureters from the underlying fibrosis or any mass and putting them in their corrected position laterally. The procedure is known as ureterolysis, and doctors advise that when medical treatment fails, double J stenting doesn’t give satisfactory results, or the presence of malignancy is suspected.
Ureterolysis can be done with an open, laparoscopic, or robotic approach, and the latter is preferred as it promises easy recovery for the patient and helps to see the internal structures of the body.
In cases where ureterolysis fails to give results, other options are ureteral reconstructive techniques (Boari flaps) and long-duration double J stents.11Hewitt, C. B., Nitz, G. L., Kiser, W. S., Straffon, R. A., & Stewart, B. H. (1969). Surgical treatment of retroperitoneal fibrosis. Annals of Surgery, 169(4), 610–615. https://doi.org/10.1097/00000658-196904000-00019
Prognosis of Retroperitoneal Fibrosis
A timely given glucocorticoid therapy for this condition promises a good prognosis. Passing a urethral catheter easily resolves symptoms of urinary obstruction. If medical treatment is not successful, the surgical approach gives favourable results. Therefore, retroperitoneal fibrosis has a good prognosis.
However, if patients also have malignancy, the life span is only about 3-6 months.12 Sinescu, I., Surcel, C., Mirvald, C., Chibelean, C., Gîngu, C., Avram, D., Hîrza, M., Manu, M., Lazar, R., Savu, C., & Udrea, A. (2010). Prognostic factors in retroperitoneal fibrosis. Journal of medicine and life, 3(1), 19–25. (Retraction published J Med Life. 2012 Jun 12;5(2):246-7.)
Can Retroperitoneal Fibrosis cause any complications?
Possible complications of this disease are:13 Brandt, A. S., Kamper, L., Kukuk, S., Haage, P., & Roth, S. (2011). Associated findings and complications of retroperitoneal fibrosis in 204 patients: results of a urological registry. The Journal of Urology, 185(2), 526–531. https://doi.org/10.1016/j.juro.2010.09.105
- Hypertension
- Anemia
- Jaundice
- Weight loss
- Nausea, vomiting
- Hydronephrosis
- Thrombosis of inferior vena cava
- Post-ureterolysis complications
Conclusion
Retroperitoneal fibrosis is a rare but serious condition that is usually not diagnosed in the early stages because its symptoms are vague and nonspecific. Most cases happen without a known cause, but autoimmune conditions, certain medications, and cancers can also cause this condition. If not diagnosed and treated on time, it can lead to complications like kidney damage or vascular problems. However, with early detection and proper treatment with steroids, most patients improve significantly. The key is timely diagnosis and consistent follow-up to avoid long-term damage.
Refrences
- 1Engelsgjerd, J. S., Leslie, S. W., & LaGrange, C. A. (2024). Retroperitoneal Fibrosis. In StatPearls. StatPearls Publishing.
- 2Runowska, M., Majewski, D., & Puszczewicz, M. (2016). Retroperitoneal fibrosis – the state-of-the-art. Reumatologia, 54(5), 256–263. https://doi.org/10.5114/reum.2016.63667
- 3Vaglio, A., & Maritati, F. (2016). Idiopathic Retroperitoneal Fibrosis. Journal of the American Society of Nephrology : JASN, 27(7), 1880–1889. https://doi.org/10.1681/ASN.2015101110
- 4Renganathan, G., Thangarasu, S., Pathak, N., Kunam, V. K., & Afzal, Z. (2024). The Enigma of Retroperitoneal Fibrosis: Clinical Implications and Diagnostic and Therapeutic Challenges. Cureus, 16(11), e74499. https://doi.org/10.7759/cureus.74499
- 5Jadhav, K. K., Kumar, V., Punatar, C. B., Joshi, V. S., & Sagade, S. N. (2017). Retroperitoneal fibrosis-clinical presentation and outcome analysis from a urological perspective. Investigative and clinical urology, 58(5), 371–377. https://doi.org/10.4111/icu.2017.58.5.371
- 6Tanaka, T., & Masumori, N. (2020). Current approach to diagnosis and management of retroperitoneal fibrosis. International journal of urology : official journal of the Japanese Urological Association, 27(5), 387–394. https://doi.org/10.1111/iju.14218
- 7Chen, S. J., Qin, L., Xie, Y. J., Zhu, J. P., Zhang, Q., & Chen, M. (2017). Ultrasonography for Preoperative Diagnosis of Retroperitoneal Fibrosis. Ultrasound quarterly, 33(2), 162–166. https://doi.org/10.1097/RUQ.0000000000000293
- 8Mulligan, S. A., Holley, H. C., Koehler, R. E., Koslin, D. B., Rubin, E., Berland, L. L., & Kenney, P. J. (1989). CT and MR imaging in the evaluation of retroperitoneal fibrosis. Journal of Computer Assisted Tomography, 13(2), 277–281. https://doi.org/10.1097/00004728-198903000-00018
- 9Wang, Y., Guan, Z., Gao, D., Luo, G., Li, K., Zhao, Y., Wang, X., Zhang, J., Jin, J., Zhao, Z., Yang, C., Zhang, J., Zhu, J., & Huang, F. (2018). The value of 18F-FDG PET/CT in the distinction between retroperitoneal fibrosis and its malignant mimics. Seminars in arthritis and rheumatism, 47(4), 593–600. https://doi.org/10.1016/j.semarthrit.2017.07.011
- 10Scheel, P. J., Jr, Sozio, S. M., & Feeley, N. (2012). Medical management of retroperitoneal fibrosis. Transactions of the American Clinical and Climatological Association, 123, 283–291.
- 11Hewitt, C. B., Nitz, G. L., Kiser, W. S., Straffon, R. A., & Stewart, B. H. (1969). Surgical treatment of retroperitoneal fibrosis. Annals of Surgery, 169(4), 610–615. https://doi.org/10.1097/00000658-196904000-00019
- 12Sinescu, I., Surcel, C., Mirvald, C., Chibelean, C., Gîngu, C., Avram, D., Hîrza, M., Manu, M., Lazar, R., Savu, C., & Udrea, A. (2010). Prognostic factors in retroperitoneal fibrosis. Journal of medicine and life, 3(1), 19–25. (Retraction published J Med Life. 2012 Jun 12;5(2):246-7.)
- 13Brandt, A. S., Kamper, L., Kukuk, S., Haage, P., & Roth, S. (2011). Associated findings and complications of retroperitoneal fibrosis in 204 patients: results of a urological registry. The Journal of Urology, 185(2), 526–531. https://doi.org/10.1016/j.juro.2010.09.105