Methanol poisoning is a serious condition that occurs due to intentional or unintentional ingestion and inhalation of methanol. The poisoning typically occurs due to errors in fermentation and distillation, or the contamination of beverages.1Nappe., J.V.A.D.H.S.T.M., Methanol Toxicity. 2025. Methanol is a poisonous alcohol that is found in several industrial and household items. Its production reached the industrial scale in 1923.2Katz, K.D., A.-M. Ruha, and S.C. Curry, Aniline and methanol toxicity after shoe dye ingestion. The Journal of Emergency Medicine, 2004. 27(4): p. 367-369.
It has extensive applications in several consumer industries, including aeroplane fuel, model cars, copy machine fluid, perfumery, and gas line antifreeze. The toxicity arises primarily from methanol’s metabolic byproduct, formic acid, which disrupts cellular respiration and leads to metabolic acidosis, optic nerve injury, and in severe cases, death. Prompt diagnosis and early treatment are critical to preventing irreversible complications. Clinical management includes supportive care, correction of acidosis, and administration of antidotes like fomepizole or ethanol, along with hemodialysis in moderate to severe cases.3Ahmed, F., et al., Methanol poisoning: 27 years’ experience at a tertiary care hospital. 2017.
Etiology and Pathophysiology
The condition can occur through:
- Methanol inhalation (e.g., from carburettor cleaners or industrial solvents)
- Methanol ingestion
- Dermal absorption of methanol (less common, but possible with prolonged exposure)
Intentional ingestion often occurs in suicide attempts or as ethanol substitution by individuals with alcohol dependency. Accidental ingestion occurs through the exploratory behaviour of young children. Common sources include windshield washer fluid, paint removers, and de-icing solutions. After ingestion, it is quickly absorbed in the gastrointestinal tract and metabolised in the liver.
The pathophysiology of methanol poisoning involves two mechanisms. These are:
Direct Central Nervous System (CNS) Depression:
Methanol acts as a CNS depressant just like ethanol. It can cause fatal CNS depression if taken in high amounts.
Toxic Metabolite Formation & Metabolic Effects:
Methanol is metabolised in the liver by the enzyme alcohol dehydrogenase (ADH) into formaldehyde. It (formaldehyde) is further metabolized by aldehyde dehydrogenase (ALDH) into formic acid (formate ion). Formic acid is the main toxic metabolite responsible for the poisoning effects, and this whole process leads to several key pathophysiological consequences. These consequences are:
- Metabolic acidosis (accumulation of formic acid)
- Inhibition of mitochondrial cytochrome C oxidase (this enzyme is inhibited by formic acid, and its inhibition causes cellular hypoxia).
- Ocular toxicity (formic acid damages the pigmented retinal epithelial cells and the optic nerve. This damage often causes blurred vision, diplopia, and photophobia, early or late blindness).
- Neurological damage (formic acid and hypoxia lead to damage in the basal ganglia, specifically the putamen. This damage causes lesions visible on imaging and possibly results in Parkinsonian-like symptoms).
- Some other systemic effects (these may include gastrointestinal symptoms, hyperglycemia, hyperkalemia, and dilated pupils).
Clinical Manifestations
Methanol poisoning presents a variety of symptoms. The initial symptoms generally appear within 12 to 24 hours after methanol ingestion. The time interval between ingestion and the occurrence of symptoms relates to the amount of ingested methanol.4Rathi, M., V. Sakhuja, and V. Jha, Visual blurring and metabolic acidosis after ingestion of bootlegged alcohol. Hemodialysis International, 2006. 10(1): p. 8-14.
Initially, the clinical manifestations of methanol poisoning are comparable to those of ethanol intoxication. In the second stage, patients may develop the following symptoms:
- Nausea
- Headache
- Vomiting
- Epigastric pain
In late stages, it can lead to :
- Drowsiness
- Obtundation
- Coma
As damage to the brain parenchyma occurs, seizures can occur in patients with methanol poisoning. They can also occur as a complication of the metabolic disturbances.
Patients primarily present with a reduced visual acuity. Patients commonly present with visual symptoms, such as blurred vision or decreased visual acuity (sharpness of vision). This may progress to scintillations (flashes of light), scotomata (blind spots in the visual field), or even complete blindness, which may or may not be reversible depending on the timing of intervention. Prompt treatment can sometimes restore vision.5Liberski, S., B.J. Kaluzny, and J. Kocięcki, Methanol-induced optic neuropathy: a still-present problem. Archives of Toxicology, 2022. 96(2): p. 431-451. Some cases have also presented the following symptoms:
- Axonal polyneuropathy6Hageman, G., et al., Parkinsonism, pyramidal signs, polyneuropathy, and cognitive decline after long-term occupational solvent exposure. Journal of Neurology, 1999. 246(3): p. 198-206.
- Amyotrophic lateral sclerosis7Chiò, A., et al., Motor neuron disease and optic neuropathy after acute exposure to a methanol‐containing solvent mixture. Amyotrophic Lateral Sclerosis and Other Motor Neuron Disorders, 2004. 5(3): p. 188-191.
Other common symptoms include:
- No breathing or breathing difficulties
- “Snowfield” vision (also called snow blindness)
- Widening of the pupils
- Blurred vision
- Low blood pressure
- Agitated behavior
- Confusion
- Dizziness
- Difficulty in walking
- Fingernails and bluish-colored lips
- Cyanosis
- Diarrhea
- Liver conditions
- Jaundice
- Pancreatitis
- Bloody vomiting
- Weakness
- Fatigue
- Leg cramps
Diagnosis of Methanol Poisoning
Diagnosis of the condition follows these steps:
History & Physical Examination:
Doctors take a careful history of high-risk patients who report typical symptoms. History is usually challenging to attain in cases of substance abuse, criminal poisoning, and self-harm. Physical findings are generally unremarkable in initial ingestions. People who have consumed methanol for self-harm typically do not want to disclose their action or feel embarrassed. Hence, the diagnostic quandary often arises. It left the clinician to consider the toxic alcohol exposures as a potential cause of the common findings (e.g., metabolic acidosis).
Patients appear mildly intoxicated after ingestion (a phase called the latent period). Symptoms of the basal ganglia can be initially concealed by altered mental status. Without treatment, the severity of illness can progress to circulatory or respiratory failure, coma, and even death.
Laboratory Diagnosis:
Laboratory diagnosis includes the following methods:
Measurement of the Serum Methanol Level
Gas chromatography is the gold standard method for the direct measurement of methanol in patients’ blood. Elevated methanol levels (>6 mmol/L or 20 mg/dL) are considered a diagnostic marker for methanol poisoning. However, toxicity may still occur at lower concentrations if formic acid has already accumulated. Elevated levels of methanol in the blood do not always correlate with clinical severity.8Anyfantakis, D., et al., Ruling in the diagnosis of methanol intoxication in a young heavy drinker: a case report. Journal of medicine and life, 2012. 5(3): p. 332.
Measurement of Serum Formate
Serum formate level can be assessed via chromatography or point-of-care formate strips. Elevated serum formate levels (19 mmol/L or above) are critical indicators.9Hovda, K.E., et al., Formate test for bedside diagnosis of methanol poisoning. Basic & clinical pharmacology & toxicology, 2021. 129(1): p. 86-88.
Blood Gas Analysis
Healthcare providers can check for arterial blood gases to assess acid-base status. The blood findings that can confirm methanol poisoning include:
- High anion gap due to the accumulation of lactate and formate.
- Base deficit
- Decreased bicarbonate
- Severe metabolic acidosis
Serum Osmolality
Ingestion of methanol results in a high osmolar gap. Hence, testing for osmolar gap is also a routine test. The osmolar gap is a generic outcome because it can represent the occurrence of a low molecular weight solute (ethanol, alcohols, glycine, mannitol, proteins).
Imaging Tests:
As the condition causes damage to the basal ganglia, and due to advanced neuroimaging techniques, this damage tends to be detected.
Computed Tomography Scanning
CT scan reveals the characteristic changes of the bilateral putaminal necrosis. It also represents the involvement of the cerebral white matter and varying degrees of hemorrhages. However, the initial CT scan is normal. Several days elapse before lesions become evident, and they may not be well-localized via CT scan as well as MRI findings.
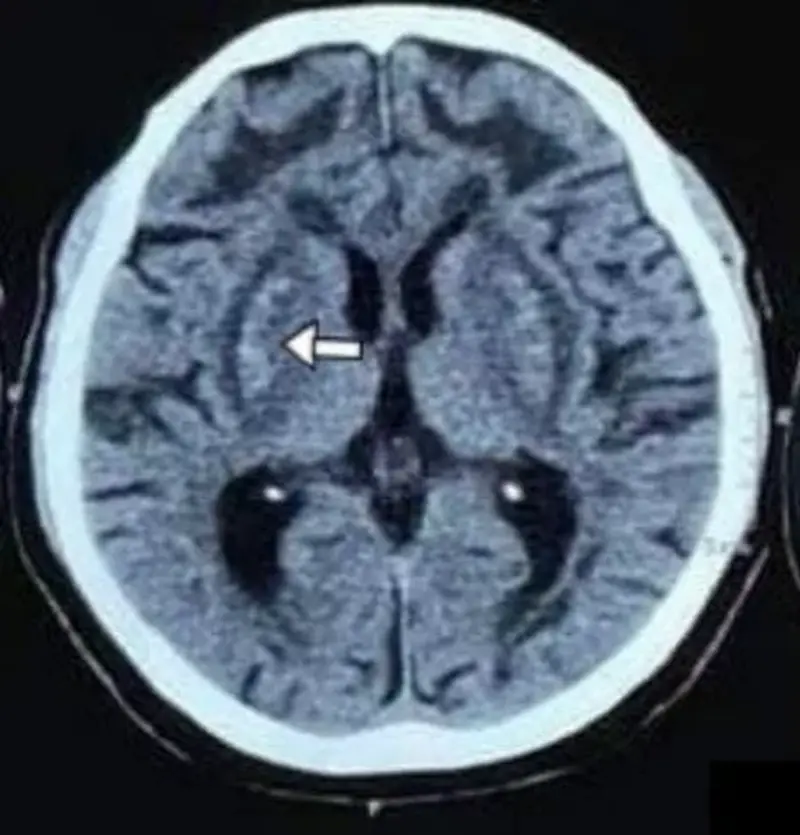
with interspersed areas of hyperdensities within the bilateral
lentiform nucleus and confluent symmetrical hypodensities
Involving deep/subcortical white matter of bilateral frontal lobes. Image courtesy: CT head findings in methanol poisoning by Shreshta et al., 2022, DOI:10.3126/jaim.v11i1.48193, available via https://www.researchgate.net/publication/363416292_CT_head_findings_in_methanol_poisoning. CC BY 4.0.
Magnetic Resonance Imaging (MRI)
MRI presents white matter lesions in the frontal or occipital lobes, diffuse cerebral edema, intraventricular hemorrhage, and enhancement of necrotic lesions and necrosis of the optic nerve. MRI is recommended as a predictive tool and a tool for differentiating methanol poisoning from other conditions (carbon monoxide poisoning and hypoglycemia).
Ophthalmologic Examination:
Performed to look for the changes such as decreased visual acuity, blurred vision, optic disc pallor or vision, visual field defects, pupillary dilation, and total blindness through visual acuity testing and fundus examination.
Treatment & Management
Immediate medical care is necessary to avoid complications. Treatment options include supportive care, antidote therapy, and hemodialysis.
Antidotes:
Some agents inhibit the toxic effects of methanol through competitive inhibition. These agents include ethanol and fomepizole.
Ethanol
Ethanol can compete with methanol for ADH. It can prevent the methanol metabolism till the methanol is eradicated from the patient’s system. ADH has a greater affinity for ethanol compared to methanol. Ethanol is intended to prevent the buildup of high formic acid levels by slowing the degradation of methanol. The goal of the ethanol treatment is to achieve an ethanol concentration of at least 100 mg/dL to prevent the metabolism of toxic alcohols. Ethanol can be administered orally or intravenously (initial IV loading dose is 600–800 mg/kg), then maintained at 66–154 mg/kg/hr, adjusted for serum levels.10Nappe., J.V.A.D.H.S.T.M., Methanol Toxicity. 2025.
Fomepizaole
It acts similarly to ethanol and is a stronger competitive inhibitor of ADH. Additionally, does not cause sedation or hypoglycemia. Administration of fomepizole is easier than ethanol as it does not require serum ethanol levels. It is administered intravenously. The loading dose of fomepizole is 15mg/kg. The maintenance dose (10 mg/kg) is administered every 12 hours until the methanol concentration drops below 25 mg/dL.11Nappe., J.V.A.D.H.S.T.M., Methanol Toxicity. 2025.
Both treatments have pros and cons. Although fomepizole is easier to dose, strongly inhibits alcohol dehydrogenase, and does not induce inebriation, it is relatively costly.
In comparison, ethanol is harder to dose precisely, requiring close monitoring of serum ethanol levels. It can induce inebriation, which necessitates intensive care monitoring, but it is less costly.
Hemodialysis:
Patients often necessitate hemodialysis even when fomepizole is administered. Also, intermittent hemodialysis is frequently favored over constant therapy as it is more effective at clearing toxic elements. Both methanol and poisonous metabolites are effectively dialysed due to their lack of protein binding, low volume distribution, and low molecular weight. This treatment is favorable even in initial methanol toxicity as it can decrease the patient’s length of stay. Hemodialysis can also lessen the risk of formate toxicity resulting from insufficient ADH inhibition. This treatment is considered in the following cases:
- Elevated anion gap
- Severe acidosis
- Seizures
- Coma
- New optical deficits
However, some patients need multiple hemodialysis sessions to lower down the methanol to non-toxic levels.
Additional Options:
Additional treatment options include supplementation with folic acid (folate). It can enhance the breakdown (metabolism) of formate to water and carbon dioxide. Its advantages are mostly hypothetical.
Supportive Therapy:
Supportive therapy is intended to initiate airway management, provide adequate hydration, and correcting electrolyte disturbances. The metabolic acidosis requires the administration of bicarbonate in addition to assisted ventilation. Bicarbonates help in decreasing the amount of active formic acid. They can also potentially reverse the visual deficits.
Differential Diagnosis
The non-toxicological considerations for the differential diagnosis of methanol poisoning include:
- Uremia
- Lactic acidosis
- Starvation ketosis
- Alcoholic ketoacidosis
- Diabetic ketoacidosis
Significant toxicological considerations (metabolic acidosis) can include poisoning through:
- Carbon monoxide
- Toluene
- Iron
- Acetaminophen
- Salicylates
Some other toxic alcohols include:
- Diethylene glycol
- Ethylene glycol
- Propylene glycol
- And isopropyl alcohol
Prognosis
Patients who receive immediate treatment are likely to have a good prognosis. Basal and retinal ganglia toxicity can also lead to permanent visual damage, Parkinsonian features, and neurological deficits. Significant morbidity and mortality can also occur due to delayed symptoms and delayed diagnosis.
Methanol Poisoning Versus Ethanol Poisoning
Methanol is more toxic than ethanol. It is mainly due to the formation of highly poisonous metabolites even after a small ingestion of methanol. Differentiating between the two is essential as they differ significantly in their management and outcomes. The key difference between these two types of poisoning is summarized in Table 1 below.
Table 1. Key Differences between Methanol and Ethanol Poisoning
|
Features |
Methanol Poisoning |
Ethanol Poisoning |
|
Sources of Poisoning |
These include:
|
These include:
|
|
Toxic Metabolites |
Formaldehyde and then formic acid. |
Acetaldehyde and then acetate. |
|
Clinical Effects |
|
|
|
Mechanism of Toxicity |
Toxic metabolites cause:
|
Directly depress the
|
|
Complications |
Complications of this condition include:
|
Complications that arise due to this condition are:
|
|
Treatment |
|
|
Methanol versus Ethylene Glycol Poisoning
Both methanol and ethylene glycol are toxic alcohols that cause poisoning, but they differ in various ways. The key differences are summarized in Table 1.
Table 1: Basic differences between Methanol versus Ethylene Glycol
| Features | Methanol Poisoning | Ethylene Glycol Poisoning |
| Sources and Exposure | Solvents, antifreeze agents, and cleaning products. | Antifreeze and industrial products |
| Symptom-onset | Dose–dependent | Usually within hours |
| Characteristics findings | Visual disturbances, snowfield, blindness, and blurred vision. | Renal failure, hypocalcemia, flank pain, and oxalate crystalluria. |
| Progression | Seizures, coma, and metabolic acidosis | Multiple organ failure. |
| Metabolic acidosis | It occurs due to formate accumulation. | It occurs due to glycolate accumulation. |
| Treatment | Folate, ethanol, fomepizole, and dialysis. | Thiamine, pyridoxine, calcium, and dialysis. |
A Quick Review
Methanol is a dangerous chemical. It can be deadly when consumed beyond its threshold. Patients who develop methanol toxicity can recover if diagnosed and treated immediately. People who are exposed to methanol gas at work should wear proper protective equipment. Any significant topical exposure or ingestion of this chemical warrants an instant call to the poison control centre. Nurses, physicians, and pharmacists should actively participate in educating the public about the proper storage of chemicals containing toxic compounds, such as methanol. It will help to ensure that they and their children are out of their reach of their toxicity. A visit to the nearby emergency department is usually recommended after suspicion of intoxication.
Refrences
- 1Nappe., J.V.A.D.H.S.T.M., Methanol Toxicity. 2025.
- 2Katz, K.D., A.-M. Ruha, and S.C. Curry, Aniline and methanol toxicity after shoe dye ingestion. The Journal of Emergency Medicine, 2004. 27(4): p. 367-369.
- 3Ahmed, F., et al., Methanol poisoning: 27 years’ experience at a tertiary care hospital. 2017.
- 4Rathi, M., V. Sakhuja, and V. Jha, Visual blurring and metabolic acidosis after ingestion of bootlegged alcohol. Hemodialysis International, 2006. 10(1): p. 8-14.
- 5Liberski, S., B.J. Kaluzny, and J. Kocięcki, Methanol-induced optic neuropathy: a still-present problem. Archives of Toxicology, 2022. 96(2): p. 431-451.
- 6Hageman, G., et al., Parkinsonism, pyramidal signs, polyneuropathy, and cognitive decline after long-term occupational solvent exposure. Journal of Neurology, 1999. 246(3): p. 198-206.
- 7Chiò, A., et al., Motor neuron disease and optic neuropathy after acute exposure to a methanol‐containing solvent mixture. Amyotrophic Lateral Sclerosis and Other Motor Neuron Disorders, 2004. 5(3): p. 188-191.
- 8Anyfantakis, D., et al., Ruling in the diagnosis of methanol intoxication in a young heavy drinker: a case report. Journal of medicine and life, 2012. 5(3): p. 332.
- 9Hovda, K.E., et al., Formate test for bedside diagnosis of methanol poisoning. Basic & clinical pharmacology & toxicology, 2021. 129(1): p. 86-88.
- 10Nappe., J.V.A.D.H.S.T.M., Methanol Toxicity. 2025.
- 11Nappe., J.V.A.D.H.S.T.M., Methanol Toxicity. 2025.