Methemoglobinemia is a blood disorder in which an abnormal amount of methemoglobin is present in the blood. MetHb is a form of hemoglobin (Hb) that has been oxidized, so the ferrous iron (Fe²⁺) is converted to ferric (Fe³⁺). Such a small, simple change impacts its ability to deliver oxygen drastically compared to hemoglobin. In methemoglobinemia, even though blood oxygen levels are actually normal, tissues do not receive oxygen. Symptoms include cyanosis, fatigue, shortness of breath, and, in severe cases, altered mental status or arrhythmias.
The first line of immediate treatment for most cases is methylene blue in an attempt to convert metHb back to functional Hb via the NADPH-dependent method. This is contraindicated in people with G6PD deficiency, whereas ascorbic acid may be used instead. The condition aggravates quickly, so immediate diagnosis and treatment can mean the difference between life and death.
Pathophysiology of Methemoglobinemia
Hemoglobin is a protein made up of 4 heme groups. Each heme contains an iron atom in the ferrous (Fe²⁺) oxidation state. When iron is ferrous, it can bind to oxygen reversibly (oxygen can bind in the lungs and release it in the tissues). When iron is oxidized to the ferric (Fe³⁺) oxidation state, it can no longer bind to oxygen. The iron in one of the heme groups of a hemoglobin protein is oxidized to the ferric state, forming a molecule called “methemoglobin”.1Ludlow JT, Wilkerson RG, Nappe TM. Methemoglobinemia. [Updated 2023 Aug 28]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK537317/
Most dangerously, it also causes the iron in the other heme groups in the same protein to bind their oxygen more tightly (a left shift in the oxygen dissociation curve), and you have less oxygen delivery to tissues.
In normal circumstances, a small amount of methemoglobin is generated and instantaneously reduced back to hemoglobin by cytochrome b5 reductase, keeping Methemoglobin levels <1%. However, when the concentration of methemoglobin outpaces the rate of re-reduction (either because of a genetic enzyme deficiency or exposure to a toxin), methemoglobinemia develops.
Thus, the body is unable to deliver oxygen to the tissues even if the blood oxygen levels are 100% normal. This is known as “functional anemia”.2Ludlow JT, Wilkerson RG, Nappe TM. Methemoglobinemia. [Updated 2023 Aug 28]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK537317/
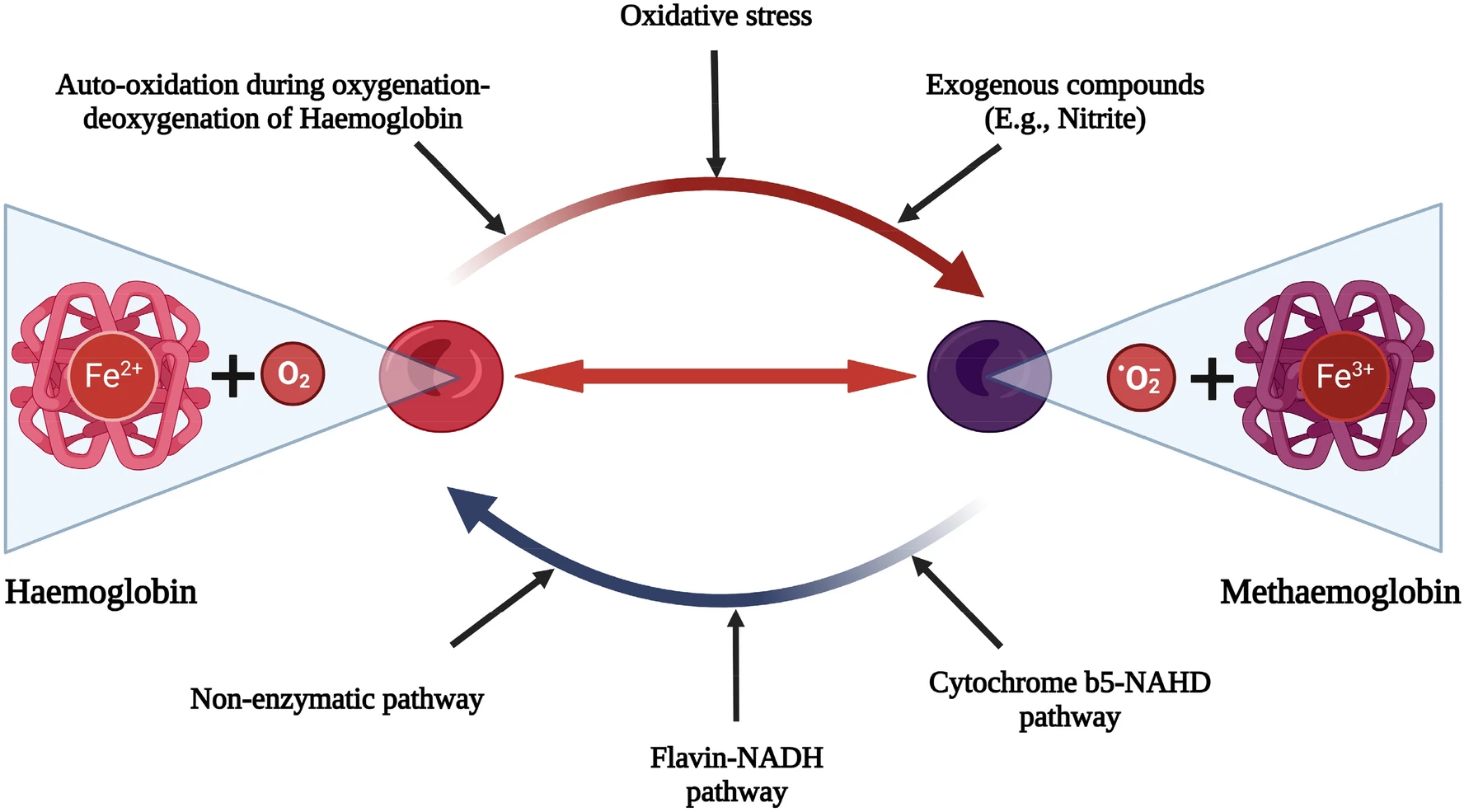
Causes of Methemoglobinemia
Methemoglobinemia can be either acquired or congenital, but the acquired form is far more common.
Acquired Methemoglobinemia:
This occurs when exposure to certain oxidizing agents overwhelms the body’s natural reductive system, leading to excessive methemoglobin formation.3Ash-Bernal, Rachel MD; Wise, Robert MD; Wright, Scott M. MD. Acquired Methemoglobinemia: A Retrospective Series of 138 Cases at 2 Teaching Hospitals. Medicine 83(5):p 265-273, September 2004. | DOI: 10.1097/01.md.0000141096.00377.3f These agents include:
- Local anesthetics (benzocaine, lidocaine, and prilocaine)
- Certain antibiotics (most commonly dapsone (used in leprosy) and sulfonamides)
- Nitrates/nitrites in contaminated water or heart medications such as nitroglycerin
- Industrial chemicals (aniline dyes, nitrobenzene)
Congenital Methemoglobinemia:
Congenital methemoglobinemia is rare (1 in 100,000 to 1 in 1,000,000 births)4Guedri, R., Missaoui, N., Essaddam, L., & Becher, S. B. (2021). A rare cause of cyanosis: Congenital methemoglobinemia. Clinical Case Reports, 9(7), e04422. https://doi.org/10.1002/ccr3.4422 and is inherited in two ways:
CYB5R (Cytochrome b5 reductase) Deficiency
- Also called NADH-dependent methemoglobin reductase5Van Zwieten, R., Verhoeven, A. J., & Roos, D. (2014). Inborn defects in the antioxidant systems of human red blood cells. Free Radical Biology and Medicine, 67, 377-386. https://doi.org/10.1016/j.freeradbiomed.2013.11.022
- Autosomal recessive
- Type I (restricted to erythrocytes) → Mild asymptomatic cyanosis (MetHb 10–35%)
- Type II (generalized) → Severe neurologic impairment, early mortality (MetHb 20–40%)
Hemoglobin M Disease
- Autosomal dominant
- Mutations in the globin chain prevent iron from being stabilized in the ferric (Fe³⁺) state
- Cyanosis with no systemic manifestations
Risk Factors of Methemoglobinemia
Factors that increase the risk of developing methemoglobinemia, depending on the context, include the following:
- Infants < 6 months are at particular risk as they have decreased levels of reductase enzyme and increased amounts of fetal hemoglobin, which is more prone to oxidation.
- G6PD deficiency increases the risk of hemolysis and can limit treatment with methylene blue.
- Nitrate exposure via well water, infant foods, or processed meats. Nitrates are also responsible for “blue baby syndrome,” a life-threatening disease causing cyanosis.
- Environmental exposure to oxidant drugs or industrial chemicals.
- Liver or kidney dysfunction impairing the detoxification of drugs.
Symptoms of Methemoglobinemia
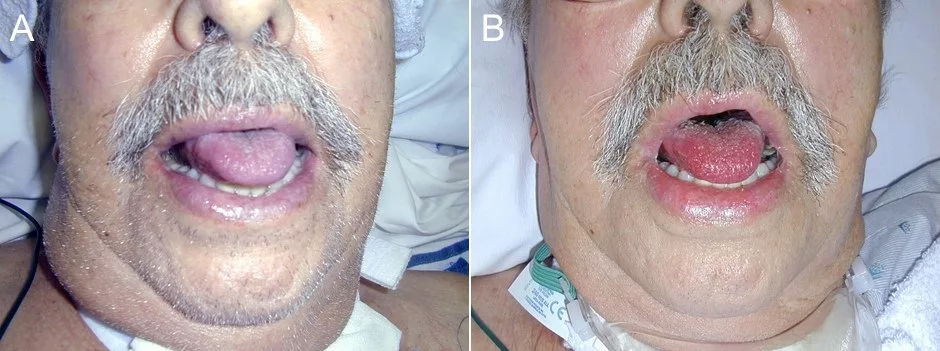
The clinical manifestations of methemoglobinemia correspond to the levels of MetHb, but the presence of cyanosis that does not improve with administration of supplemental oxygen, particularly in a patient who has been receiving any of the implicated drugs or has a relevant exposure history, should be a big red flag to the clinician.
| MetHb Level | Signs and Symptoms6Iolascon, A., Bianchi, P., Andolfo, I., Russo, R., Barcellini, W., Fermo, E., Toldi, G., Ghirardello, S., Rees, D., Wijk, R. V., Kattamis, A., Gallagher, P. G., Roy, N., Taher, A., Mohty, R., Kulozik, A., Franceschi, L. D., Gambale, A., Montalembert, M. D., . . . Prchal, J. (2021). Recommendations for diagnosis and treatment of methemoglobinemia. American Journal of Hematology, 96(12), 1666. https://doi.org/10.1002/ajh.26340 |
|---|---|
| <10–15% | Asymptomatic or mild cyanosis (lips, nail beds) |
| 15–30% | Visible cyanosis (skin, lips, mucosa), “chocolate-colored” blood |
| 30–50% | Fatigue, dyspnea, headache, dizziness, tachycardia |
| 50–70% | Confusion, lethargy, seizures, metabolic acidosis |
| >70% | Severe hypoxia, cardiovascular collapse, life-threatening complications, death |
Diagnosis of Methemoglobinemia
Initial Suspicion:
- A clinician should be suspicious in case of any patient who presents with cyanosis not responsive to 100% oxygen.
- However, cyanotic congenital heart diseases like Tetralogy of Fallot should also be considered, especially in pediatric patients.
- Other observations that can add to the suspicion are:
- Chocolate-brown blood that does not turn red on air exposure
- Recent history of exposure to oxidizing agents (e.g., local anesthetics, nitrates, dapsone)
- Sudden onset of dyspnea, fatigue, or altered mental state without an obvious pulmonary or cardiac cause
Confirmatory Testing:
Diagnosis of methemoglobinemia is mostly clinical. The confirmatory tests are quite simple and quick.
- Pulse Oximetry (SpO₂): SpO₂ is low (~85% or less) and does not change significantly with oxygen administration. This is because pulse oximetry uses infrared, and infrared lights are absorbed differently by oxygenated and deoxygenated hemoglobin. This is why the SpO2 readings are inaccurate in the presence of methemoglobin.
- Arterial Blood Gases (ABGs): PaO₂ is usually normal since dissolved oxygen is unaffected. This helps rule out hypoxic respiratory failure as the primary cause of cyanosis.
- Saturation Gap: When SpO₂ on pulse oximetry is significantly lower than PaO₂ in ABGs, it’s considered a ‘saturation gap’ and a dyshemoglobinemia is suspected. Keep in mind that a saturation gap is a key clue, but not diagnostic on its own.
- Even when a saturation gap is identified, co-oximetry is needed to confirm the presence and type of dyshemoglobinemia (e.g., methemoglobinemia vs. carboxyhemoglobinemia). It’s the only test that can accurately differentiate them.
- Co-oximetry: This is the gold standard diagnostic test because it directly measures MetHb levels7Nitzan, M., Romem, A., & Koppel, R. (2014). Pulse oximetry: Fundamentals and technology update. Medical Devices (Auckland, N.Z.), 7, 231. https://doi.org/10.2147/MDER.S47319, along with other hemoglobin types like carboxyhemoglobin and oxyhemoglobin, and is available on most modern blood gas analyzers. MetHb concentration >1–2% is abnormal, and symptoms usually appear when levels exceed 10–15%.
Additional Investigations:
- CBC, renal function, and liver function testing to evaluate for comorbid conditions, complications, particularly if the event is recurrent.
- G6PD levels may be helpful before methylene blue use, particularly in patients of African, Mediterranean, and South Asian descent.
- Genetic testing to evaluate for CYB5R3 mutations (congenital methemoglobinemia Type I/II) or hemoglobin M evaluation (HBA1/HBA2, HBB) if the event is recurrent or if the etiology remains unknown.
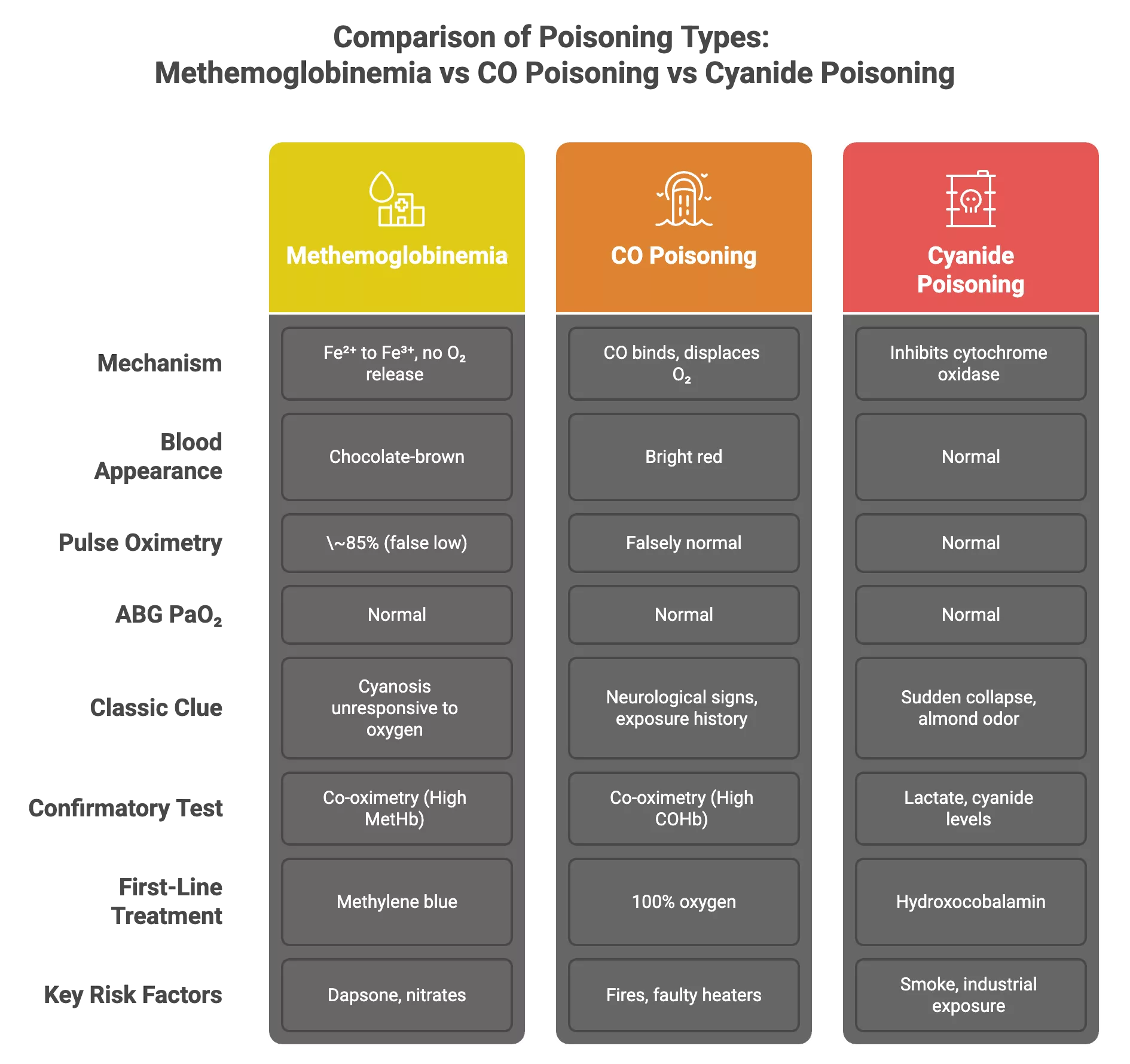
Methemoglobinemia vs. Carbon Monoxide Poisoning vs. Cyanide Poisoning
These three conditions are distinct in mechanism, but they all have the same effect on the body: poor oxygen delivery or utilization at the tissue level, even though oxygen levels in the blood are normal. Methemoglobinemia and carbon monoxide poisoning8Chenoweth, J. A., Albertson, T. E., & Greer, M. R. (2021). Carbon Monoxide Poisoning. Critical care clinics, 37(3), 657–672. https://doi.org/10.1016/j.ccc.2021.03.010 are both forms of dyshemoglobinemia that can be differentiated by co-oximetry.
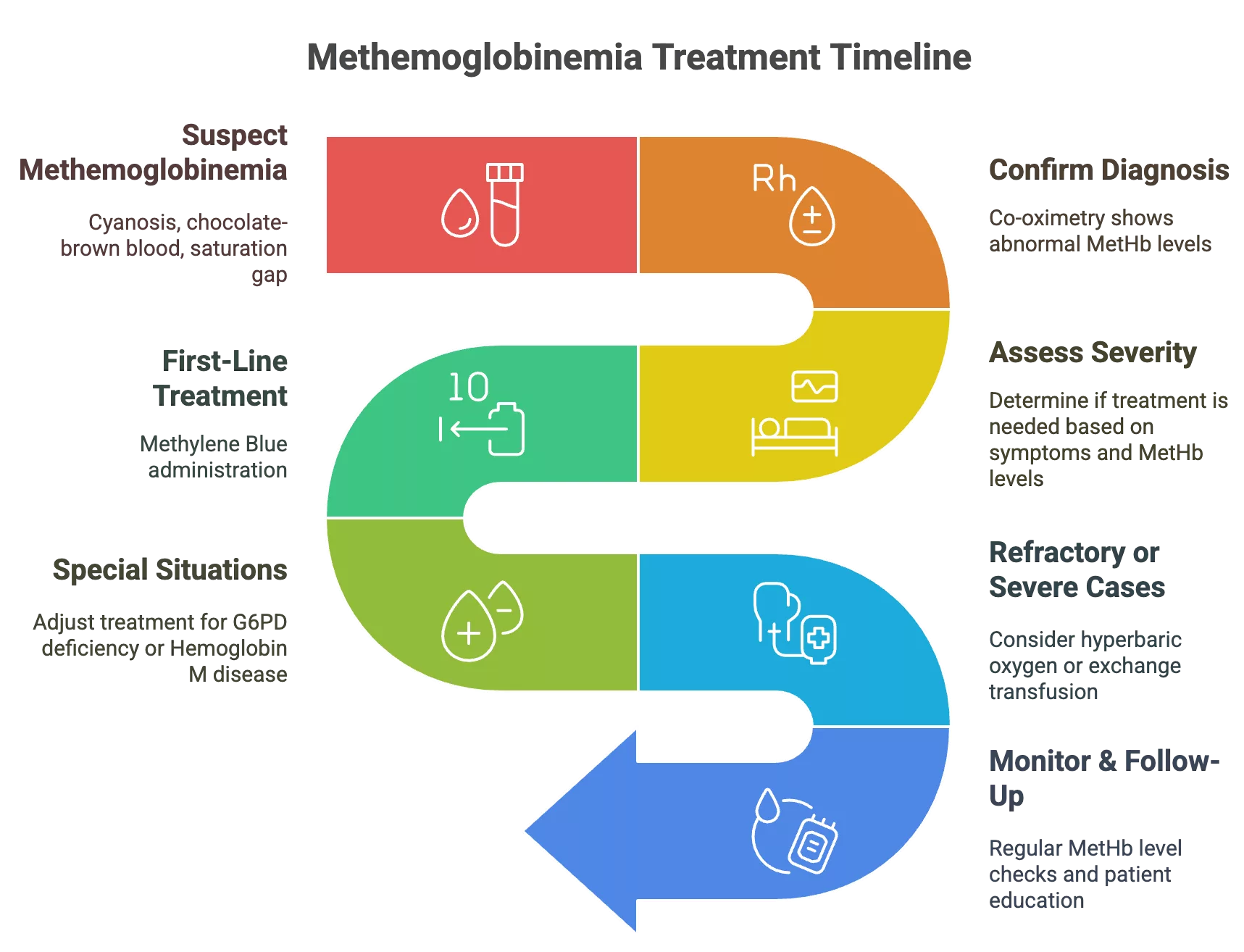
Treatment & Management of Methemoglobinemia
The management plan for methemoglobinemia depends on the MetHb levels and the extent of symptoms. Mild, asymptomatic elevations may not require immediate intervention, but symptomatic or rapidly progressing cases need to be treated urgently.
Initial Steps:
- Immediately stop the causative agent/drug
- Provide supplemental oxygen, even though SpO₂ on pulse oximetry will not improve, it still helps with tissue oxygenation.
Acute Management Protocol:
First-Line Treatment
- Methylene blue is indicated as the first-line antidote in symptomatic patients or those with MetHb levels over 20%.9Pushparajah Mak, R. S., & Liebelt, E. L. (2021). Methylene Blue: An Antidote for Methemoglobinemia and Beyond. Pediatric emergency care, 37(9), 474–477. https://doi.org/10.1097/PEC.0000000000002526
- Dose: 1–2 mg/kg is administered intravenously over 5 minutes.10Bistas E, Sanghavi DK. Methylene Blue. [Updated 2023 Jun 26]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK557593/
- The dose may be repeated after an hour if no improvement is seen (max dose in total: 7 mg/kg)
- Methylene blue serves as an electron donor via the NADPH-dependent pathway, reducing MetHb back to hemoglobin
- It is contraindicated in G6PD deficiency (risk of hemolysis, can lead to acute hemolytic anemia)11Palmer, K., Dick, J., French, W., Floro, L., & Ford, M. (2020). Methemoglobinemia in Patient with G6PD Deficiency and SARS-CoV-2 Infection. Emerging Infectious Diseases, 26(9), 2279. https://doi.org/10.3201/eid2609.202353 and Hemoglobin M disease (ineffective).
Second-Line Options
- Ascorbic Acid (Vitamin C): 300–600 mg/day PO or IV. It is a slow reducer of MetHb, and is only preferred in chronic, congenital cases or when methylene blue is contraindicated
- Exchange Transfusion: Severe cases unresponsive to methylene blue or patients with G6PD deficiency benefit from blood transfusions.
- Hyperbaric (high-pressure) oxygen can temporarily improve oxygen delivery to tissues. It should be considered in life-threatening situations, especially when other therapies are contraindicated or ineffective
Monitoring:
- It’s important to reassess MetHb levels every 1–2 hours after treatment.
- Monitoring is focused on rebound methemoglobinemia, especially with long-acting agents like dapsone.
- Renal function should be evaluated before repeating methylene blue doses, even within allowed amounts.
Prevention and Prognosis of Methemoglobinemia:
Prognosis:
The prognosis of methemoglobinemia is favorable if treatment is sought timely manner:
- Acquired cases respond well to methylene blue within a few hours if treatment is started early. In severe cases or when treatment is delayed, it can lead to seizures, arrhythmias, coma, or death, especially when MetHb exceeds 50–70%.
- Congenital forms often cause lifelong cyanosis, but with appropriate management and patient knowledge about known triggers, there are no major systemic effects.
Prevention & Long-Term Care of Methemoglobinemia:
- In high-risk populations like infants, high-nitrate water sources (>10 ppm) should be avoided, introduction of high-nitrate vegetables such as spinach in an infant’s diet should be delayed, and teething gels that contain benzocaine should not be used.
- In cases with G6PD or CYB5R3 deficiency, patient education can be helpful, so that they know to avoid triggers like dapsone, sulfonamides, nitrites, aniline dyes, etc.
- In patients with a congenital form of the disease, genetic counseling should be offered, and the family should be screened to identify other high-risk individuals.
Conclusion
Methemoglobinemia can be life-threatening and requires high suspicion and prompt intervention. Its hallmark finding: cyanosis that does not respond to oxygen, should always raise clinical suspicion, especially with recent oxidant exposure or otherwise unexplained symptoms. With the correct diagnostic tests and evaluation, particularly in the utilization of co-oximetry, emergency medicine experts can rapidly determine the accurate diagnosis to provide methylene blue or other treatments to reverse hypoxemia.
Ultimately, prevention of methemoglobinemia can be achieved by medication safety, public water supply vigilance, and high-risk patient screening for genetic susceptibility. Increased awareness among providers and patients about this easily missed diagnosis can lead to rapid identification, decreasing the incidence, and improving outcomes for both acute and chronic presentations.
Refrences
- 1Ludlow JT, Wilkerson RG, Nappe TM. Methemoglobinemia. [Updated 2023 Aug 28]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK537317/
- 2Ludlow JT, Wilkerson RG, Nappe TM. Methemoglobinemia. [Updated 2023 Aug 28]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK537317/
- 3Ash-Bernal, Rachel MD; Wise, Robert MD; Wright, Scott M. MD. Acquired Methemoglobinemia: A Retrospective Series of 138 Cases at 2 Teaching Hospitals. Medicine 83(5):p 265-273, September 2004. | DOI: 10.1097/01.md.0000141096.00377.3f
- 4Guedri, R., Missaoui, N., Essaddam, L., & Becher, S. B. (2021). A rare cause of cyanosis: Congenital methemoglobinemia. Clinical Case Reports, 9(7), e04422. https://doi.org/10.1002/ccr3.4422
- 5Van Zwieten, R., Verhoeven, A. J., & Roos, D. (2014). Inborn defects in the antioxidant systems of human red blood cells. Free Radical Biology and Medicine, 67, 377-386. https://doi.org/10.1016/j.freeradbiomed.2013.11.022
- 6Iolascon, A., Bianchi, P., Andolfo, I., Russo, R., Barcellini, W., Fermo, E., Toldi, G., Ghirardello, S., Rees, D., Wijk, R. V., Kattamis, A., Gallagher, P. G., Roy, N., Taher, A., Mohty, R., Kulozik, A., Franceschi, L. D., Gambale, A., Montalembert, M. D., . . . Prchal, J. (2021). Recommendations for diagnosis and treatment of methemoglobinemia. American Journal of Hematology, 96(12), 1666. https://doi.org/10.1002/ajh.26340
- 7Nitzan, M., Romem, A., & Koppel, R. (2014). Pulse oximetry: Fundamentals and technology update. Medical Devices (Auckland, N.Z.), 7, 231. https://doi.org/10.2147/MDER.S47319
- 8Chenoweth, J. A., Albertson, T. E., & Greer, M. R. (2021). Carbon Monoxide Poisoning. Critical care clinics, 37(3), 657–672. https://doi.org/10.1016/j.ccc.2021.03.010
- 9Pushparajah Mak, R. S., & Liebelt, E. L. (2021). Methylene Blue: An Antidote for Methemoglobinemia and Beyond. Pediatric emergency care, 37(9), 474–477. https://doi.org/10.1097/PEC.0000000000002526
- 10Bistas E, Sanghavi DK. Methylene Blue. [Updated 2023 Jun 26]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK557593/
- 11Palmer, K., Dick, J., French, W., Floro, L., & Ford, M. (2020). Methemoglobinemia in Patient with G6PD Deficiency and SARS-CoV-2 Infection. Emerging Infectious Diseases, 26(9), 2279. https://doi.org/10.3201/eid2609.202353

