Myelofibrosis (MF) is a complex bone marrow disorder in which scar tissue (fibrosis) replaces the healthy bone marrow, disrupting blood cell production. It falls under a group of diseases known as myeloproliferative neoplasms (MPNs). In all MPNs, the basic defect is an abnormal growth of blood-forming cells (marrow stem cells).1Barbui, T., Thiele, J., Gisslinger, H., Kvasnicka, H. M., Vannucchi, A. M., Guglielmelli, P., Orazi, A., & Tefferi, A. (2018). The 2016 WHO classification and diagnostic criteria for myeloproliferative neoplasms: Document summary and in-depth discussion. Blood Cancer Journal, 8(2), 15. https://doi.org/10.1038/s41408-018-0054-y In myelofibrosis, this abnormality leads to anemia, severe fatigue, and an enlarged spleen.
Myelofibrosis can develop in two ways:
- Primary myelofibrosis: This occurs on its own due to genetic changes.
- Secondary myelofibrosis: This results from other MPNs, such as polycythemia vera (PV) or essential thrombocythemia (ET).
Myelofibrosis develops slowly and can lead to life-threatening complications, such as bone marrow failure or acute leukemia, a more aggressive type of blood cancer. In recent years, early diagnosis and new treatments have improved outcomes, offering patients better disease management and quality of life.
Causes & Risk Factors
The exact cause is not known. In nearly 90% of the cases, myelofibrosis is driven by genetic mutations in bone marrow stem cells. 60% of the patients have mutations in the JAK2 gene, 20-30% have mutations in the CALR gene and 5-10% have mutations in the MPL gene. When mutations are not present in any of the former genes in about 10-15% of the patient population, then the disease is labeled as “Triple-negative Myelofibrosis”.2Mascarenhas, J., Gleitz, H. F. E., Chifotides, H. T., Harrison, C. N., Verstovsek, S., Vannucchi, A. M., Rampal, R. K., Kiladjian, J. J., Vainchenker, W., Hoffman, R., Schneider, R. K., & List, A. F. (2023). Biological drivers of clinical phenotype in myelofibrosis. Leukemia, 37(2), 255–264. https://doi.org/10.1038/s41375-022-01767-y
Who is at risk of developing Myelofibrosis?
What causes the above-mentioned mutations in the bone marrow cells is unknown. However, conditions and factors that may favor these mutations are:
- Age (most patients are over 50)
- Existing blood disorders (like other myeloproliferative neoplasms)
- Exposure to high radiation or industrial chemicals (e.g., benzene).
Is Myelofibrosis hereditary?
No. The disease is not directly inherited but there are families having increased risk towards myeloproliferative neoplasms and related blood cancers, pointing towards some common genetic factor predisposing them.
How does Myelofibrosis develop?
Healthy bone marrow consists of stem cells that develop into red blood cells, which carry oxygen, white blood cells, which help detect and fight infection and platelets, which are cells that help blood to clot. Myelofibrosis starts at the cellular level by genetic mutations in stem cells which make them function abnormally. Marker proteins are released by these cells which instigate inflammation, leading to myelofibrosis or scarring of the bone marrow and abnormal blood cell formation leading to abnormal blood cell counts.3Fisher, D. A. C., Fowles, J. S., Zhou, A., & Oh, S. T. (2021). Inflammatory Pathophysiology as a Contributor to Myeloproliferative Neoplasms. Frontiers in immunology, 12, 683401. https://doi.org/10.3389/fimmu.2021.683401
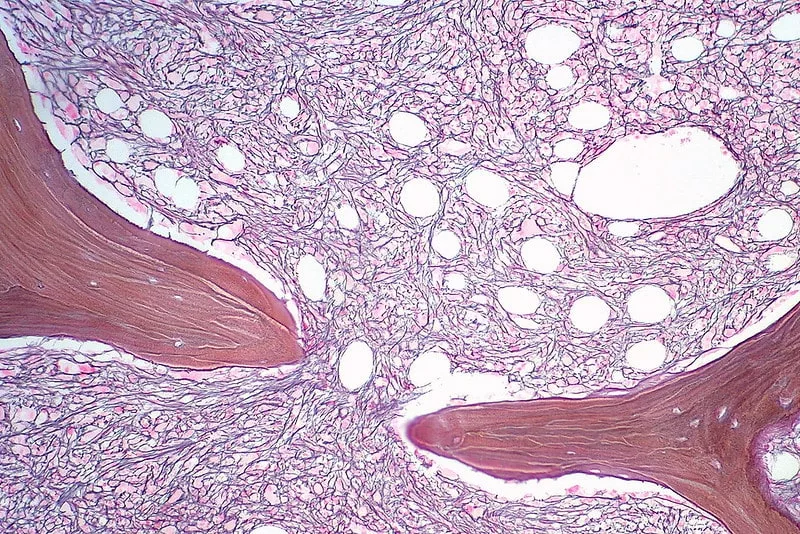
Symptoms of Myelofibrosis
Myelofibrosis often starts with symptoms that can be mistaken for general fatigue. The earliest signs include unexplained fatigue, easy bruising, frequent nosebleeds, night sweats, and general discomfort.
As myelofibrosis progresses, symptoms become more noticeable because of bone marrow failure, abnormal blood cell production, and organ enlargement. The most common symptoms include:
- Severe fatigue & weakness due to anemia (low red blood cell count).
- Pain or fullness in the left upper abdomen because of an enlarged spleen (splenomegaly) and enlarged liver (hepatomegaly) as they take over blood cell production (extra-medullary hematopoiesis).4Oon, S.F., Singh, D., Tan, T.H. et al. Primary myelofibrosis: spectrum of imaging features and disease-related complications. Insights Imaging 10, 71 (2019). https://doi.org/10.1186/s13244-019-0758-y
- Unintentional weight loss often linked to increased metabolism due to disease activity.
- Easy bruising & bleeding due to low platelet levels (thrombocytopenia), making clotting difficult.
- Swollen gums or nosebleeds from poor clotting.
- Purplish spots (petechiae) on the skin due to fragile blood vessels and low platelet count.
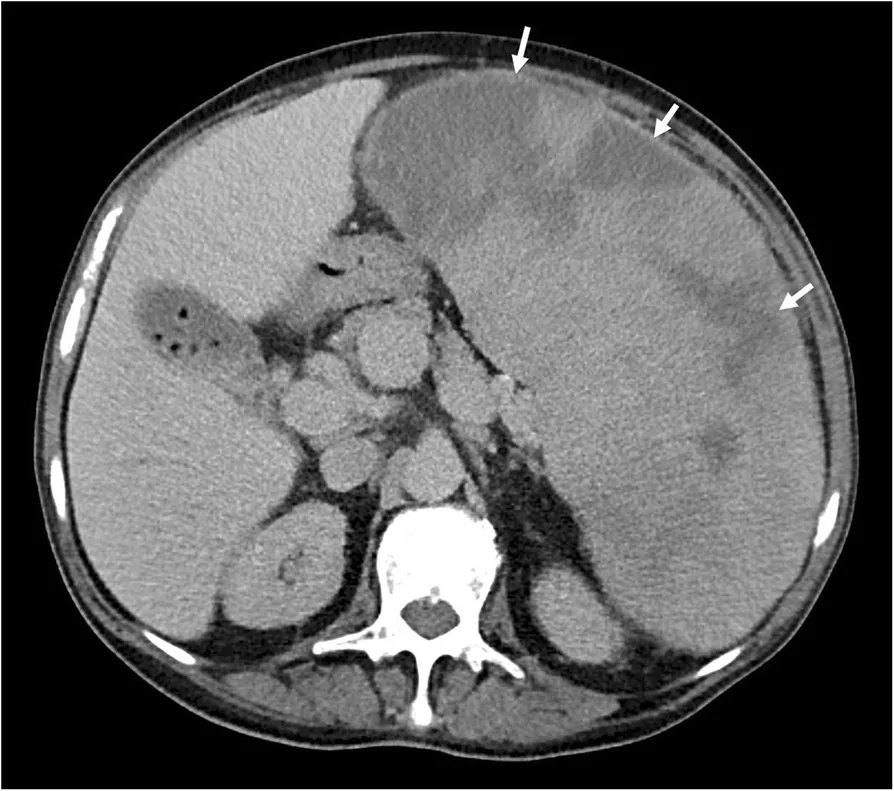
Complications of Myelofibrosis
In the later phases of the disease, there can be severe anemia requiring frequent blood transfusions, portal hypertension, intense itching (pruritus), and bone marrow failure leading to dangerously low blood counts and increased risk of infections.
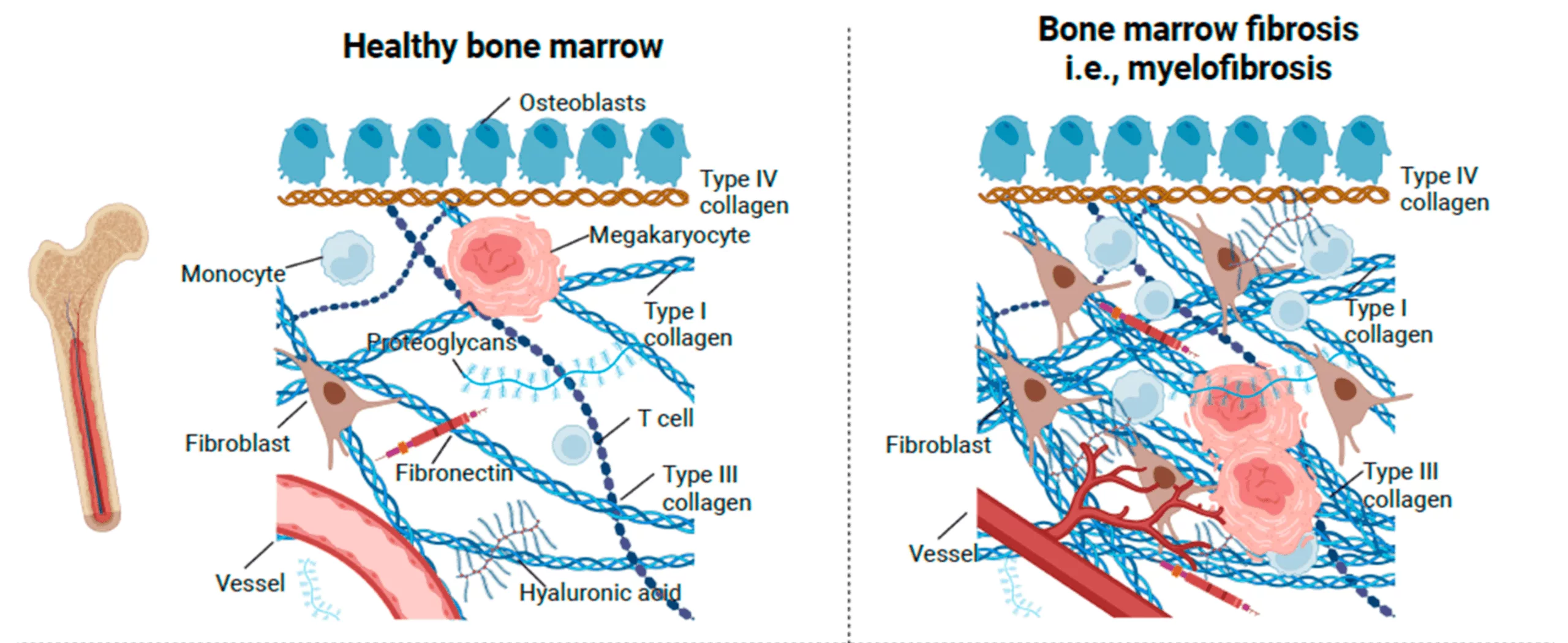
(Adapted from Hasselbalch, H. C., Junker, P., Skov, V., Kjær, L., Knudsen, T. A., Larsen, M. K., Holmström, M. O., Andersen, M. H., Jensen, C., Karsdal, M. A., & Willumsen, N. (2023). Revisiting Circulating Extracellular Matrix Fragments as Disease Markers in Myelofibrosis and Related Neoplasms. Cancers, 15(17), 4323. Available at MDPI. Licensed under CC BY 4.0.)
Can Myelofibrosis turn into Leukemia?
Yes. About 10-20% of primary MF patients progress to leukemia, mostly acute myeloid leukemia (AML). Rare instances of lymphoblastic transformation have also been observed. Genetic testing helps predict this risk.5Dunbar, A. J., Rampal, R. K., & Levine, R. (2020). Leukemia secondary to myeloproliferative neoplasms. Blood, 136(1), 61–70. https://doi.org/10.1182/blood.2019000943
Diagnosis of Myelofibrosis
Since myelofibrosis shares symptoms with other conditions, the diagnosis involves a combination of tests and imaging.
- Blood Tests:
- Complete Blood Count (CBC) – Checks for abnormal red and white blood cell levels and platelet counts.
- Peripheral Blood Smear – Examines blood cell shape and abnormalities. Teardrop-shaped red blood cells and immature blood cells are key features of the disease.
- Bone Marrow Biopsy: A sample of bone marrow is taken (usually from the hip) to check for fibrosis (scarring) and abnormal cell production.
- Genetic Testing: Identifies mutations in genes like JAK2, CALR, or MPL, which are commonly linked to myelofibrosis.
- Imaging Scans: Ultrasound or MRI to assess spleen and liver enlargement.
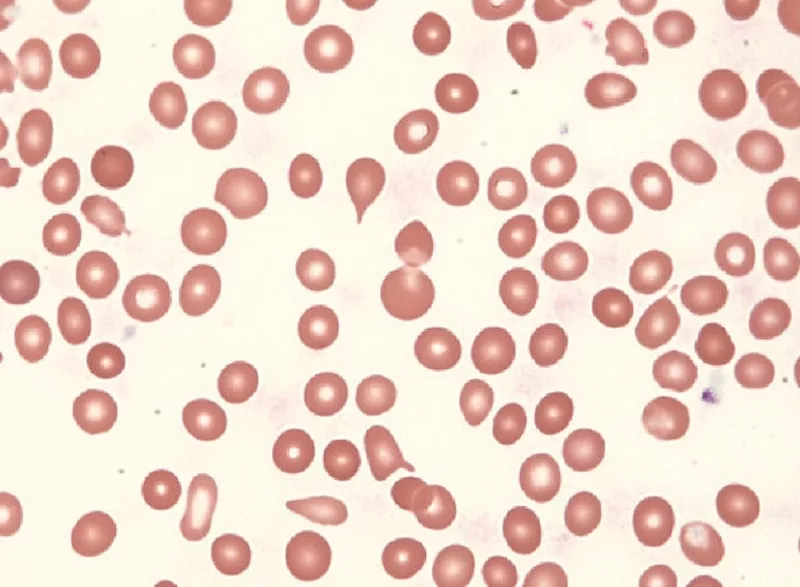
Up to 30% of patients are asymptomatic at diagnosis, with abnormalities detected incidentally during routine blood tests. The World Health Organization (WHO) diagnostic criteria require bone marrow fibrosis plus specific genetic or clinical features.6Tefferi A. (2021). Primary myelofibrosis: 2021 update on diagnosis, risk-stratification and management. American journal of hematology, 96(1), 145–162. https://doi.org/10.1002/ajh.26050
Since the symptoms of myelofibrosis can overlap with other conditions, early testing is key to catching myelofibrosis in its initial stages. If you notice persistent fatigue, unexplained bruising, night sweats, or unexplained weight loss, consulting a doctor is advised.
Stages of Myelofibrosis
Myelofibrosis is not staged like other cancers. Instead, it is risk-stratified. Risk stratification is a way to determine your risk level and to help guide a provider in the options that may be best for you given your risk factors. Risk stratifications use different systems. The most widely used system currently is MIPSS70+ 2.0 (Mutation-Enhanced International Prognostic Scoring System).7Guglielmelli, P., Lasho, T. L., Rotunno, G., Mudireddy, M., Mannarelli, C., Nicolosi, M., Pacilli, A., Pardanani, A., Rumi, E., Rosti, V., Hanson, C. A., Mannelli, F., Ketterling, R. P., Gangat, N., Rambaldi, A., Passamonti, F., Barosi, G., Barbui, T., Cazzola, M., … Tefferi, A. (2018). MIPSS70: Mutation-enhanced international prognostic score system for transplantation-age patients with primary myelofibrosis. Journal of Clinical Oncology, 36(4), 310-318. https://doi.org/10.1200/JCO.2017.76.4886 This system uses both genetic and clinical factors to determine risks and stage patients into one of five groups:
| Risk Classification | What It Means | Median Survival (Prognosis) |
|---|---|---|
| Very Low Risk | No major symptoms. | ~15+ years |
| Low Risk | Some symptoms, but the disease is still mild. | ~10-15 years |
| Intermediate-1 | Symptoms become more noticeable; some impact on daily life. | ~7-10 years |
| Intermediate-2 | Significant symptoms, worsening anemia, higher leukemia risk. | ~4-7 years |
| High Risk | Severe symptoms, high chance of disease progression. | ~2-4 years |
Treatment & Management
There is no known cure for myelofibrosis—management depends on the severity of symptoms, disease progression, and individual factors.
Low-Risk Patients (Asymptomatic)
If symptoms are mild or absent, regular monitoring with blood tests and imaging is recommended. No immediate treatment is necessary unless the disease progresses.
High-Risk or Symptomatic Patients
- Some drugs, like JAK Inhibitors, can help reduce spleen size, alleviate symptoms, and improve quality of life.
- Blood transfusions and drugs that can stimulate the production of red blood cells (RBCs) can help manage anemia.
- For severe splenomegaly unresponsive to drugs, splenectomy or radiation therapy may work.
Bone marrow transplantation (also called stem cell transplant) is the only curative option, but it is reserved for younger, healthier patients due to high risks.
What new treatments or clinical trials are available?
Research is being done on new JAK inhibitors, gene therapy, and combination drug treatments. Patients may choose to participate in a clinical trial if they are not able to achieve remission with the existing treatments.8Tremblay, D., Yacoub, A., & Hoffman, R. (2021). Overview of Myeloproliferative Neoplasms: History, Pathogenesis, Diagnostic Criteria, and Complications. Hematology/oncology clinics of North America, 35(2), 159–176. https://doi.org/10.1016/j.hoc.2020.12.001

Can lifestyle changes help manage Myelofibrosis?
Many patients are able to live with their disease in relatively good condition for many years. Taking the following steps may help to attain the best possible quality of life and symptom control:
- Balanced Nutrition: Eating foods rich in iron, lean meats, plus fruits and vegetables can assist in overall health.
- Regular Exercise: Light to moderate activity, like walking or yoga, can increase strength and overall well-being.
- Managing Stress: Meditation, therapy, or relaxation techniques can help.
- Prevent Infections: Vaccinations like getting a yearly flu shot, etc, and good hygiene practices should be used because of a suppressed immune system.
Life Expectancy
There is an average life expectancy of 3 to 11 years following diagnosis. However, some patients may live much longer if they have lower-risk disease, and others may develop rapid progression with more high-risk features.9Mora, B., Bucelli, C., Cattaneo, D. et al. Prognostic and Predictive Models in Myelofibrosis. Curr Hematol Malig Rep 19, 223–235 (2024). https://doi.org/10.1007/s11899-024-00739-6
Can Myelofibrosis be cured?
At this time, the only potential cure is a stem cell transplant, which involves serious risks including transplant-related mortality, graft-versus-host disease (GVHD), and infection.10Robin, M., de Wreede, L. C., Wolschke, C., Schetelig, J., Eikema, D. J., Van Lint, M. T., Knelange, N. S., Beelen, D., Brecht, A., Niederwieser, D., Vitek, A., Bethge, W., Arnold, R., Finke, J., Volin, L., Yakoub-Agha, I., Nagler, A., Poiré, X., Einsele, H., Chevallier, P., … Kröger, N. (2019). Long-term outcome after allogeneic hematopoietic cell transplantation for myelofibrosis. Haematologica, 104(9), 1782–1788. https://doi.org/10.3324/haematol.2018.205211 It is usually considered for patients younger than 65 years old who have an HLA-matched donor. For the majority of other patients, the goal of therapy is to improve symptoms and slow the course of the disease.
Myelofibrosis vs Myelodysplastic Syndrome:
| Myelofibrosis (MF) | Myelodysplastic Syndrome (MDS)11Garcia-Manero G. Myelodysplastic syndromes: 2023 update on diagnosis, risk-stratification, and management. Am J Hematol. 2023; 98(8): 1307-1325. doi:10.1002/ajh.26984 | |
|---|---|---|
| Cause | Myelofibrosis can cause fibrosis or scarring in the bone marrow leading to the inability of the bone marrow to produce normal blood cells. | Bone marrow produces abnormal, poorly functioning blood cells due to genetic mutations. |
| Main Effect on Blood Cells | Myelofibrosis can cause fibrosis or scarring in the bone marrow, leading to the inability of the bone marrow to produce normal blood cells. | Low levels of all blood cells (anemia, low platelets, low white cells). |
| Symptoms | Fatigue, night sweats, enlarged spleen, easy bruising. | Low red blood cells (anemia), abnormal white blood cells, and an enlarged spleen. |
| Progression | Can slowly worsen over time, sometimes progressing to acute leukemia. | Can remain stable or progress to acute myeloid leukemia (AML). |
| Treatment | It can slowly worsen over time, sometimes progressing to acute leukemia. | JAK inhibitors, chemotherapy, and stem cell transplant for high-risk cases. |
Conclusion
Myelofibrosis is a life-altering disease, but survival and quality of life can be improved with early diagnosis and treatment. Unless you obtain a stem cell transplant, which is a small percentage of patients, most patients will not be cured. However, treatment can help improve symptoms and slow the progression of the disease, so many patients can live productive lives. If you or a loved one is diagnosed with myelofibrosis, it is important that you continue to read, consult your health care provider, and get involved with a myeloproliferative neoplasm group.
Refrences
- 1Barbui, T., Thiele, J., Gisslinger, H., Kvasnicka, H. M., Vannucchi, A. M., Guglielmelli, P., Orazi, A., & Tefferi, A. (2018). The 2016 WHO classification and diagnostic criteria for myeloproliferative neoplasms: Document summary and in-depth discussion. Blood Cancer Journal, 8(2), 15. https://doi.org/10.1038/s41408-018-0054-y
- 2Mascarenhas, J., Gleitz, H. F. E., Chifotides, H. T., Harrison, C. N., Verstovsek, S., Vannucchi, A. M., Rampal, R. K., Kiladjian, J. J., Vainchenker, W., Hoffman, R., Schneider, R. K., & List, A. F. (2023). Biological drivers of clinical phenotype in myelofibrosis. Leukemia, 37(2), 255–264. https://doi.org/10.1038/s41375-022-01767-y
- 3Fisher, D. A. C., Fowles, J. S., Zhou, A., & Oh, S. T. (2021). Inflammatory Pathophysiology as a Contributor to Myeloproliferative Neoplasms. Frontiers in immunology, 12, 683401. https://doi.org/10.3389/fimmu.2021.683401
- 4Oon, S.F., Singh, D., Tan, T.H. et al. Primary myelofibrosis: spectrum of imaging features and disease-related complications. Insights Imaging 10, 71 (2019). https://doi.org/10.1186/s13244-019-0758-y
- 5Dunbar, A. J., Rampal, R. K., & Levine, R. (2020). Leukemia secondary to myeloproliferative neoplasms. Blood, 136(1), 61–70. https://doi.org/10.1182/blood.2019000943
- 6Tefferi A. (2021). Primary myelofibrosis: 2021 update on diagnosis, risk-stratification and management. American journal of hematology, 96(1), 145–162. https://doi.org/10.1002/ajh.26050
- 7Guglielmelli, P., Lasho, T. L., Rotunno, G., Mudireddy, M., Mannarelli, C., Nicolosi, M., Pacilli, A., Pardanani, A., Rumi, E., Rosti, V., Hanson, C. A., Mannelli, F., Ketterling, R. P., Gangat, N., Rambaldi, A., Passamonti, F., Barosi, G., Barbui, T., Cazzola, M., … Tefferi, A. (2018). MIPSS70: Mutation-enhanced international prognostic score system for transplantation-age patients with primary myelofibrosis. Journal of Clinical Oncology, 36(4), 310-318. https://doi.org/10.1200/JCO.2017.76.4886
- 8Tremblay, D., Yacoub, A., & Hoffman, R. (2021). Overview of Myeloproliferative Neoplasms: History, Pathogenesis, Diagnostic Criteria, and Complications. Hematology/oncology clinics of North America, 35(2), 159–176. https://doi.org/10.1016/j.hoc.2020.12.001
- 9Mora, B., Bucelli, C., Cattaneo, D. et al. Prognostic and Predictive Models in Myelofibrosis. Curr Hematol Malig Rep 19, 223–235 (2024). https://doi.org/10.1007/s11899-024-00739-6
- 10Robin, M., de Wreede, L. C., Wolschke, C., Schetelig, J., Eikema, D. J., Van Lint, M. T., Knelange, N. S., Beelen, D., Brecht, A., Niederwieser, D., Vitek, A., Bethge, W., Arnold, R., Finke, J., Volin, L., Yakoub-Agha, I., Nagler, A., Poiré, X., Einsele, H., Chevallier, P., … Kröger, N. (2019). Long-term outcome after allogeneic hematopoietic cell transplantation for myelofibrosis. Haematologica, 104(9), 1782–1788. https://doi.org/10.3324/haematol.2018.205211
- 11Garcia-Manero G. Myelodysplastic syndromes: 2023 update on diagnosis, risk-stratification, and management. Am J Hematol. 2023; 98(8): 1307-1325. doi:10.1002/ajh.26984

