What is Pearson Syndrome?
Pearson Syndrome is a rare, fatal mitochondrial disorder characterized by multisystem involvement, commonly affecting the bone marrow and pancreas. With an incidence of approximately one in every million people,1Farruggia, P., Di Cataldo, A., Pinto, R. M., Palmisani, E., Macaluso, A., Valvo, L. L., Cantarini, M. E., Tornesello, A., Corti, P., Fioredda, F., Varotto, S., Martire, B., Moroni, I., Puccio, G., Russo, G., Dufour, C., & Pillon, M. (2016). Pearson Syndrome: A Retrospective Cohort Study from the Marrow Failure Study Group of A.I.E.O.P. (Associazione Italiana Emato-Oncologia Pediatrica). JIMD reports 26, 37–43. https://doi.org/10.1007/8904_2015_470 it is exceptionally rare. Most patients die in early childhood because of lactic acidosis and organ failure. Due to the overlapping manifestations with various hematological and metabolic diseases, it is frequently misdiagnosed and underdiagnosed, posing a substantial diagnostic challenge for healthcare providers.
Pathophysiology of Pearson Syndrome
Pearson syndrome is a primary mitochondrial disorder characterized by mutations in the mitochondrial DNA (mtDNA). Mitochondria, often referred to as the cell’s powerhouse, play a pivotal role in energy generation through oxidative phosphorylation. Crucial proteins and enzymes encoded by mtDNA drive this process. However, in individuals with Pearson syndrome, disruptions occur as a result of mtDNA mutations, leading to defective protein synthesis and impaired oxidative phosphorylation.

Consequently, the mitochondria become incapable of producing adenosine triphosphate (ATP), the cell’s primary energy source. This deficiency in ATP production results in the malfunctioning of the organs dependent on this energy currency. For instance, impaired ATP synthesis in hematopoietic (blood-forming) cells of the bone marrow leads to deficiencies in blood cells, manifesting as anemias, recurrent infections, and bleeding disorders. Similarly, inadequate ATP synthesis in pancreatic beta cells results in insulin deficiency, manifesting as diabetes mellitus.
Overall, mitochondrial dysfunction secondary to mtDNA mutations contributes to multi-organ involvement, explaining the diverse clinical manifestations of Pearson syndrome.
Causes of Pearson Syndrome
The primary causes of Pearson syndrome involve deletions, rearrangements, and duplications in mitochondrial DNA (mtDNA). Common mutations include2Rötig, A., Cormier, V., Koll, F., Mize, C. E., Saudubray, J. M., Veerman, A., Pearson, H. A., & Munnich, A. (1991). Site-specific deletions of the mitochondrial genome in the Pearson marrow-pancreas syndrome. Genomics, 10(2), 502–504. https://doi.org/10.1016/0888-7543(91)90342-c deletions affecting genes such as:
- ATPases 6 and 8
- Cytochrome c oxidase III
- NADH dehydrogenase 3, 4, 4L, and 5
There is some evidence of a potential link between the urea cycle and the underlying pathology of Pearson syndrome. Studies have shown that while ammonia levels in Pearson syndrome patients are generally normal, levels of citrulline and arginine, crucial components of the urea cycle, are significantly reduced. This has led researchers to hypothesize that certain substrates of the urea cycle may be redirected toward nucleotide synthesis in the body.3Crippa, B. L., Leon, E., Calhoun, A., Lowichik, A., Pasquali, M., & Longo, N. (2015). Biochemical abnormalities in Pearson syndrome. American journal of medical genetics. Part A, 167A(3), 621–628. https://doi.org/10.1002/ajmg.a.36939
Variability in Presentation
While most cases result in early mortality, some individuals with mosaicism or less severe mtDNA mutations may survive longer. These patients often develop secondary mitochondrial disorders, such as Kearns-Sayre Syndrome, in later life. This highlights the variability in disease progression and underscores the importance of early diagnosis and supportive care.4Yoshimi A, Ishikawa K, Niemeyer C, Grünert SC. Pearson syndrome: a multisystem mitochondrial disease with bone marrow failure. Orphanet J Rare Dis. 2022 Oct 17;17(1):379. doi: 10.1186/s13023-022-02538-9. PMID: 36253820; PMCID: PMC9575259.
Inheritance of Pearson Syndrome
The majority of cases of Pearson Syndrome (PS) result from de novo mutations in mitochondrial DNA (mtDNA). These mutations occur spontaneously during fertilization or early embryonic development rather than being inherited from a parent. This explains why most affected individuals do not have a family history of the disorder.
In rare instances, Pearson Syndrome can follow a mitochondrial inheritance pattern, where mutations in mtDNA are passed from an affected mother to her child. Mitochondrial inheritance is exclusively maternal, as mitochondria—and thus mtDNA—are inherited solely from the mother. However, this mode of transmission is uncommon in PS. The number of mutated mtDNA copies passed from mother to child during cell division is usually insufficient to cause the syndrome. Consequently, predicting whether a child of an affected mother will develop PS is difficult and depends on the proportion of mutated mtDNA (heteroplasmy) transmitted.5Bernes, S. M., et al. (1993). Identical mitochondrial DNA deletion in mother with progressive external ophthalmoplegia and son with Pearson marrow-pancreas syndrome. The Journal of Pediatrics, 123(4), 598–602. https://doi.org/10.1016/s0022-3476(05)80962-x[/mfn]
Symptoms of Pearson Syndrome
The symptoms of Pearson syndrome vary according to the organs involved and the age of presentation. Common symptoms include:
Neonates:
In the neonatal period, the majority of babies remain asymptomatic. The symptomatic 40% usually present with:
This atypical presentation of PS in the neonatal period makes it difficult to diagnose and is often overlooked
Infancy or Early Childhood:
The patients in infancy or early childhood exhibit the following symptoms:
Later in Life
The few who survive early childhood land into neurological and muscular abnormalities such as Leigh syndrome and Kearns-Sayre syndrome.
In the study mentioned earlier, researchers discovered that 64% of babies initially tested normal at birth but later experienced speech delay, muscle wasting, and weakness. Additionally, 11% of these babies eventually displayed all symptoms associated with Kearns-Sayre syndrome.5Farruggia, P., Di Cataldo, A., Pinto, R. M., Palmisani, E., Macaluso, A., Valvo, L. L., Cantarini, M. E., Tornesello, A., Corti, P., Fioredda, F., Varotto, S., Martire, B., Moroni, I., Puccio, G., Russo, G., Dufour, C., & Pillon, M. (2016). Pearson Syndrome: A Retrospective Cohort Study from the Marrow Failure Study Group of A.I.E.O.P. (Associazione Italiana Emato-Oncologia Pediatrica). JIMD reports 26, 37–43. https://doi.org/10.1007/8904_2015_470
Diagnosis of Pearson Syndrome
After taking a thorough history and performing a complete physical examination, the doctor orders the following lab tests to reach the diagnosis of Pearson syndrome:
Initial Workup:
Blood Complete Picture with Differential & Reticulocyte Count
- Due to bone marrow failure, all blood cell lines are underproduced, which manifests as macrocytic anemia, leukopenia, neutropenia, and thrombocytopenia.
- As the bone marrow is out of order, the reticulocyte counts are also low.
Urine Analysis
Loss of amino acids, glucose, bicarbonate, phosphate, citrate, and urate in the urine (renal tubular dysfunction) is characteristic.
Fecal Elastase
Elastase is an enzyme produced by the exocrine pancreas that helps digest carbohydrates and proteins. In Pearson Syndrome, as there is a problem with our pancreas, it does not produce enough elastase. This is why the levels of elastase enzyme, when measured in stool, are below the normal range.
Liver Function Tests
- Whenever there is damage to the liver, the liver cells also known as the hepatocytes, release a few enzymes such as Aspartate Aminotransferase (AST) and Alanine Aminotransferase (ALT). This is the reason why patients of PS might also have elevated levels of these liver enzymes in their blood.
- Our liver is the production house for many proteins that help our blood to clot (also known as clotting factors). Therefore, PS also manifests as a deranged clotting profile during lab investigations.
Serum Lactate & Pyruvate Levels
You will see an increase in serum lactate levels. This is because as there is a disturbance of aerobic metabolism at the level of mitochondria, the cells’ metabolism shifts towards the anaerobic path. This type of metabolism yields lactate as a by-product, as compared to pyruvate, which is produced in aerobic metabolism. Due to the same reason, there is an elevation of the lactate-to-pyruvate ratio at the baseline.
Endocrinological Study
Thyroid function tests, growth hormone levels, parathyroid hormone levels, adrenal hormone levels, etc might be deranged when the disease affects these endocrine glands.
Confirmatory Tests:
Lab tests in the initial workup strengthen the suspicion of PS, which is then confirmed by the following investigations:
Bone Marrow Aspiration & Biopsy
This investigation reveals pathognomonic signs of PS, which are:
- Hypocellular bone marrow
- Vacuolization of myeloid and erythroid precursor cells
- Presence of ring-sideroblasts
Once the hematologist identifies these findings in BM biopsy, the healthcare team will proceed toward cytogenetic analysis(analysis of the chromosomes and the DNA) to confirm the diagnosis.
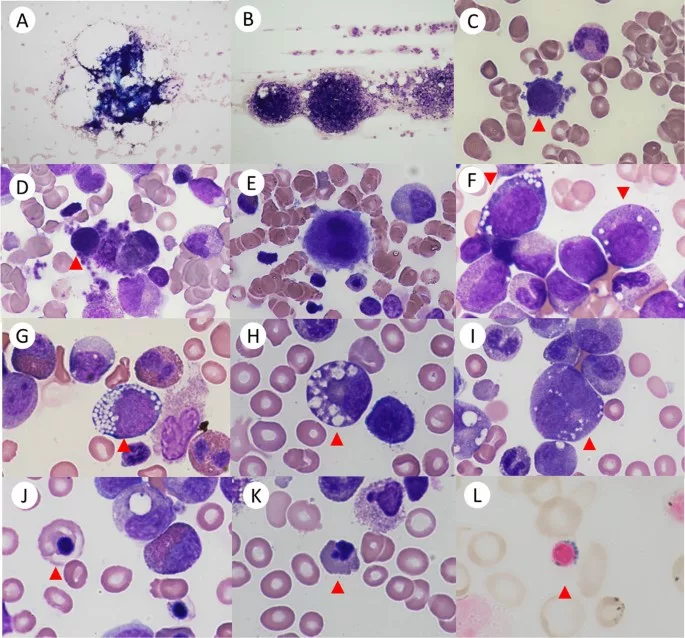
Image Courtesy: Yoshimi, A., Ishikawa, K., Niemeyer, C. et al. Pearson syndrome: a multisystem mitochondrial disease with bone marrow failure. Orphanet J Rare Dis 17, 379 (2022). https://doi.org/10.1186/s13023-022-02538-9 available under license CC BY 4.0
Genetic Testing
Genetic analysis of mtDNA from blood, bone, or buccal mucosa is the gold standard investigation. The presence of a single, large-scale mitochondrial DNA deletion is sufficient to establish its diagnosis.
Differential Diagnoses:
Other disorders that resemble Pearson syndrome include:
- Bone Marrow Failure: It can be ruled out by an absence of mtDNA deletion on genetic testing
- Diamond Blackfan anemia: The patients have raised serum adenosine levels and no pancreatic insufficiency.
- Fanconi Anemia: The anemia in this disease is not accompanied by pancreatic insufficiency. Patients usually present with a deficiency of platelets before anemia.
- Shwachman-Diamond Syndrome: The most characteristic finding is neutropenia instead of macrocytic anemia
- Myelodysplastic Syndrome (MDS): While this syndrome also exhibits vacuolization of progenitor blood cells, additional cytogenetic abnormalities are present as well.
- Neutropenia
- Nutritional Considerations in Failure to Thrive
- Kearns-Sayre Syndrome
- MELAS Syndrome
The doctor will rule these out based on comprehensive history, thorough examination, and relevant investigations.
Treatment of Pearson Syndrome
The treatment of Pearson syndrome involves a multidisciplinary team that includes a hematologist, an endocrinologist, an expert in genetics and metabolism, a gastroenterologist, and other specialists based on the patient’s clinical manifestations.
Since there is no definitive cure available to date, the treatment of Pearson syndrome revolves around improving the quality of life and reducing morbidity as much as possible:
- Red cell transfusions and erythropoietin for anemia.
- Pancreatic enzyme replacement and supplementation with fat-soluble vitamins to manage dysfunction of the exocrine pancreas
- Antibiotics and G-CSF(granulocyte colony-stimulating factor)6Baertling, F., Meissner, T., Troeger, A., Pillekamp, F., Mayatepek, E., Laws, H. J., & Distelmaier, F. (2014). Granulocyte colony-stimulating factor for the treatment of neutropenia-associated infection in Pearson syndrome. Klinische Padiatrie, 226(3), 190–191. https://doi.org/10.1055/s-0034-1368760 for infections and neutropenia
- Stem cell transplantation7Faraci, M., Cuzzubbo, D., Micalizzi, C., Lanino, E., Morreale, G., Dallorso, S., Castagnola, E., Schiaffino, M. C., Bruno, C., Rossi, A., Dini, G., & Cappelli, B. (2007). Allogeneic bone marrow transplantation for Pearson’s syndrome. Bone marrow transplantation, 39(9), 563–565. https://doi.org/10.1038/sj.bmt.1705638 to treat bone marrow failure
- Diabetes and other endocrinopathies should be treated accordingly if present
- Hydration, correction of electrolyte abnormalities and acidosis for managing metabolic crisis
- Appropriate support for those with neuromuscular manifestations
Prognosis of Pearson Syndrome
The prognosis of Pearson syndrome is often poor. Most patients do not make it past the age of six.8Reynolds, E., Byrne, M., Ganetzky, R., & Parikh, S. (2021). Pediatric single large-scale mtDNA deletion syndromes: The power of patient-reported outcomes. Molecular genetics and metabolism, 134(4), 301–308. https://doi.org/10.1016/j.ymgme.2021.11.004
In a retrospective study by Anteneová et al. (2020), researchers found that the 5-year survival rate of patients with Pearson syndrome is 60%, which is lower compared to 100% for those with other mitochondrial diseases.
Common causes of death in Pearson syndrome include:
- Lactic acidosis during metabolic crises
- Hepatic failure
- Sepsis due to neutropenia (deficiency in white blood cells)
- These findings highlight the serious nature of Pearson syndrome and the challenges in managing its complications.
Conclusion
Pearson Syndrome is a deadly mitochondrial disease that is often misdiagnosed owing to its rarity and similarity with other diseases. The majority of patients do not survive infancy or childhood. Common clinical manifestations include hematological abnormalities, malabsorption, liver failure, and neuromuscular problems. For a healthcare provider, it is crucial to rule out the differentials and reach the correct diagnosis as early as possible to minimize fatality and improve the quality of life for the patient.
Refrences
- 1Farruggia, P., Di Cataldo, A., Pinto, R. M., Palmisani, E., Macaluso, A., Valvo, L. L., Cantarini, M. E., Tornesello, A., Corti, P., Fioredda, F., Varotto, S., Martire, B., Moroni, I., Puccio, G., Russo, G., Dufour, C., & Pillon, M. (2016). Pearson Syndrome: A Retrospective Cohort Study from the Marrow Failure Study Group of A.I.E.O.P. (Associazione Italiana Emato-Oncologia Pediatrica). JIMD reports 26, 37–43. https://doi.org/10.1007/8904_2015_470
- 2Rötig, A., Cormier, V., Koll, F., Mize, C. E., Saudubray, J. M., Veerman, A., Pearson, H. A., & Munnich, A. (1991). Site-specific deletions of the mitochondrial genome in the Pearson marrow-pancreas syndrome. Genomics, 10(2), 502–504. https://doi.org/10.1016/0888-7543(91)90342-c
- 3Crippa, B. L., Leon, E., Calhoun, A., Lowichik, A., Pasquali, M., & Longo, N. (2015). Biochemical abnormalities in Pearson syndrome. American journal of medical genetics. Part A, 167A(3), 621–628. https://doi.org/10.1002/ajmg.a.36939
- 4Yoshimi A, Ishikawa K, Niemeyer C, Grünert SC. Pearson syndrome: a multisystem mitochondrial disease with bone marrow failure. Orphanet J Rare Dis. 2022 Oct 17;17(1):379. doi: 10.1186/s13023-022-02538-9. PMID: 36253820; PMCID: PMC9575259.
- 5Bernes, S. M., et al. (1993). Identical mitochondrial DNA deletion in mother with progressive external ophthalmoplegia and son with Pearson marrow-pancreas syndrome. The Journal of Pediatrics, 123(4), 598–602. https://doi.org/10.1016/s0022-3476(05)80962-x[/mfn]
Symptoms of Pearson Syndrome
The symptoms of Pearson syndrome vary according to the organs involved and the age of presentation. Common symptoms include:
- Pallor and fatigue: Due to anemia
- Infections: Due to deficiency of white blood cells
- Easy bruising and bleeding: Due to deficient platelets
- Malabsorption, liver steatosis: Due to impaired function of the exocrine pancreas
- Diabetes: Due to dysfunction of pancreatic insulin-producing beta cells
- Renal and/or adrenal insufficiency
- Liver failure
- Droopy eyelids, vision problems, hearing loss, seizures, or movement disorders
Neonates:
In the neonatal period, the majority of babies remain asymptomatic. The symptomatic 40% usually present with:
- Persistent anemia
- Low birth weight
- Microcephaly
- Multi-organ failure
Infancy or Early Childhood:
The patients in infancy or early childhood exhibit the following symptoms:
- Failure to thrive
- Chronic diarrhea
- Hepatomegaly
- Metabolic crises characterized by somnolence, vomiting, electrolytic abnormalities, lactic acidosis, and hepatic insufficiency
Later in Life
The few who survive early childhood land into neurological and muscular abnormalities such as Leigh syndrome and Kearns-Sayre syndrome.
In the study mentioned earlier, researchers discovered that 64% of babies initially tested normal at birth but later experienced speech delay, muscle wasting, and weakness. Additionally, 11% of these babies eventually displayed all symptoms associated with Kearns-Sayre syndrome.5Farruggia, P., Di Cataldo, A., Pinto, R. M., Palmisani, E., Macaluso, A., Valvo, L. L., Cantarini, M. E., Tornesello, A., Corti, P., Fioredda, F., Varotto, S., Martire, B., Moroni, I., Puccio, G., Russo, G., Dufour, C., & Pillon, M. (2016). Pearson Syndrome: A Retrospective Cohort Study from the Marrow Failure Study Group of A.I.E.O.P. (Associazione Italiana Emato-Oncologia Pediatrica). JIMD reports 26, 37–43. https://doi.org/10.1007/8904_2015_470 - 6Baertling, F., Meissner, T., Troeger, A., Pillekamp, F., Mayatepek, E., Laws, H. J., & Distelmaier, F. (2014). Granulocyte colony-stimulating factor for the treatment of neutropenia-associated infection in Pearson syndrome. Klinische Padiatrie, 226(3), 190–191. https://doi.org/10.1055/s-0034-1368760
- 7Faraci, M., Cuzzubbo, D., Micalizzi, C., Lanino, E., Morreale, G., Dallorso, S., Castagnola, E., Schiaffino, M. C., Bruno, C., Rossi, A., Dini, G., & Cappelli, B. (2007). Allogeneic bone marrow transplantation for Pearson’s syndrome. Bone marrow transplantation, 39(9), 563–565. https://doi.org/10.1038/sj.bmt.1705638
- 8Reynolds, E., Byrne, M., Ganetzky, R., & Parikh, S. (2021). Pediatric single large-scale mtDNA deletion syndromes: The power of patient-reported outcomes. Molecular genetics and metabolism, 134(4), 301–308. https://doi.org/10.1016/j.ymgme.2021.11.004

