Castleman disease (CD) refers to a rare, nonclonal lymphoproliferative disorder characterized by abnormal overgrowth of lymph node tissue. Lymph nodes are small, bean-shaped structures that help the body fight infection. Although it is not cancerous, it can behave in ways that mimic both malignant and benign conditions, often presenting as masses in the chest, neck, abdomen, or pelvis. CD is classified into subtypes based on both histological appearance and clinical presentation.
Clinically, CD is divided into two main forms: the more common unicentric Castleman disease (UCD), which affects a single group of lymph nodes, and the less common but more severe multicentric Castleman disease (MCD), which involves multiple lymph node regions and is often associated with systemic symptoms such as fever, fatigue, and weight loss. Its pathological and histological classification includes plasma cell type, hyaline vascular type (HV-CD), human herpesvirus (HHV)-8 associated CD, and mixed type CD.
The incidence of UCD is 16 patients per million per year. The prevalence of HHV-8-associated CD varies widely, while the incidence of MCD is five per million yearly and affects people of all age groups.1Ehsan, N. and F. Zahra, Castleman disease. 2022.
Types of Castleman Disease
The following are common types of CDs:
Unicentric CD:
UCD involves a single enlarged lymph node or a single group of lymph nodes. Although often asymptomatic, some individuals may experience symptoms due to the mass effect of the enlarged node. Histologically, the affected node shows Castleman-like features, typically falling along a spectrum.2El Hussein, S., et al., Unicentric Castleman disease: illustration of its morphologic Spectrum and review of the differential diagnosis. Archives of Pathology & Laboratory Medicine, 2024. 148(1): p. 99-106.
These characteristics include:
- Enlarged or atrophic germinal centers
- Increased vascularity (hypervascularity)
- Polyclonal plasmacytosis (presence of many plasma cells)
- Expansion of polyclonal T and B lymphocytes
- Prominence of follicular dendritic cells (FDC)
The histopathological subtypes of the UCD include:
Plasma-cell
Plasma cell UCD involves:
- Hyperplastic germinal centers
- Occasional atrophic germinal centers
- Interfolicular plasmacytosis
Hyaline Vascular Type (HV-CD)
This type presents:
- Small, atrophic germinal centers with “onion-skinning” mantle zones
- Predominance of FDC
- Hypervascularization
- Strong membrane staining in germinal centers
- Negative staining in the mantle zone3Talat, N., A.P. Belgaumkar, and K.-M. Schulte, Surgery in Castleman’s disease: a systematic review of 404 published cases. Annals of Surgery, 2012. 255(4): p. 677-684.

Mixed Type CD
Mixed-type CD depicts features of both plasma cell subtypes and hyaline vascular type.
Multicentric CD:
MCD involves multiple lymph node regions and is often accompanied by systemic inflammatory symptoms such as fever, fatigue, weight loss, enlarged liver or spleen, and laboratory abnormalities. The multicentric CD further includes the following types:
HHV-8 Associated MCD
This form occurs due to uncontrolled infection with human herpesvirus-8 (HHV-8), particularly in individuals with HIV or other immunocompromised states. HHV-8 drives overproduction of pro-inflammatory cytokines, especially interleukin-6 (IL-6).4Yu, L., et al., Clinical and pathological characteristics of HIV- and HHV-8–and HHV-8-negative Castleman disease. Blood, The Journal of the American Society of Hematology, 2017. 129(12): p. 1658-1668.
People suffering from HHV-8-associated MCD are at high risk of developing other conditions, such as:
- Hodgkin Lymphoma
- Non-Hodgkin lymphoma
- Kaposi sarcoma
Idiopathic MCD (iMCD)
HHV-8 negative or idiopathic MCD involves multiple regions of the enlarged lymph nodes with CD-like characteristics. iMCD patients are also negative for HIV. Researchers reported elevated IL-6 and cytokine levels. The exact cause of the disease is not known, but the hypothesized causes include inherited mutations that lead to autoinflammation and autoimmunity, as well as acquired mutations in a clonal cell population or a pathogen.5Lang, E. and F. van Rhee, Idiopathic multicentric Castleman disease: an update in diagnosis and treatment advances. Blood Reviews, 2024. 64: p. 101161.
There are three further subtypes of IMCD:
iMCD-Associated with TAFRO:
TAFRO is named for its associated symptoms. The symptoms include thrombocytopenia (low platelets), anasarca (swelling from fluid build-up), fever, reticulin fibrosis, or renal dysfunction organomegaly syndrome (TAFRO), which sometimes accompanies iMCD. These cases show normal gamma globulin levels and mixed or hypervascular, previously called hyaline vascular histopathological characteristics.
iMCD with Idiopathic Plasmacytic Lymphadenopathy (iMCD-IPL):
This variant is characterized by:
- Abundant plasma cells in lymph nodes
- Thrombocytosis (elevated platelet count)
- Hypergammaglobulinemia (high levels of antibodies in the blood)
POEMS-Associated HHV-8-negative MCD:
In this form, multicentric Castleman disease coexists with POEMS syndrome, a rare disorder defined by:
- Polyneuropathy
- Organomegaly
- Endocrinopathy
- Monoclonal plasma cell disorder
- Skin changes
Causes of Castleman Disease
There are very few facts about the causes of this disease. However, some evidence suggests that CDs can result from impaired immunoregulation, causing abundant proliferation of B lymphocytes and plasma cells in lymphoid organs. Such conditions occur from
- Viral infections
- Lymphoid-hamartomatous
- Chronic low-grade inflammation
- Angiogenesis
- Abnormal modulation of cytokines6Han, X. and D.-B. Zhou, Advances in etiology and management of Castleman’s disease. Zhongguo yi xue ke xue Yuan xue bao. Acta Academiae Medicinae Sinicae, 2009. 31(5): p. 639-643.
Researchers have discovered a close association between the disease and HIV, which indicates immunodeficiency. Other causative factors, such as human herpesvirus-8 (HHV-8), cause deregulation of inflammatory mediators like interleukin (IL)-6 and CD20. All cases of HIV CD are HHV-8 positive compared to 40-50% of non-HIV-CD. Other cases unrelated to HHV-8 or HIV, termed idiopathic multicentric CD (iMCD), still have no established etiology.7Ehsan, N. and F. Zahra, Castleman disease. 2022.
Symptoms of Castleman Disease
UCD has milder symptoms and rarely affects the vital organs (kidneys, liver, and bone marrow). It can present the following symptoms:
- Enlargement of a single lymph node
- Enlargement of a single region of the lymph nodes
- Flue-like illness
The presentation of the MCD spans a wide spectrum of severity, from mild to life-threatening organ failure. The common symptoms include:
- Flue-like illness
- Fever
- Night sweats
- Fatigue
- Unexplained weight loss
- Nausea
- Vomiting
- Swelling in your abdomen
- Swelling in your ankles or feet
- Numbness in your hands or feet
- Abnormal blood counts
- Organ failure8Van Rhee, F., A. Greenway, and K. Stone, Treatment of idiopathic Castleman disease. Hematology/Oncology Clinics, 2018. 32(1): p. 89-106.
Diagnosis of Castleman Disease
CD diagnosis involves a series of clinical evaluations, imaging, and lymph node biopsy.
History & Physical Evaluations:
The doctors will inquire about your medical history, symptoms, and related conditions. In the case of UCD, patients present a slow-growing and non-malignant painless solitary mass at the anatomic site.9Wang, S., et al., Clinicopathological characteristics of unicentric retroperitoneal Castleman’s disease: a study of 14 cases. World Journal of Surgical Oncology, 2015. 14: p. 1-4.
MCD presentation may also have three or more of the following:
- Cough
- Rash
- Ascites
- Edema
- Splenomegaly
- Xerostomia
- Nasal obstruction
- Peripheral lymphadenopathy
- Autoimmune hemolytic anemia
- Jaundice
- Pleural effusion
- Splenomegaly
- Central nervous system symptoms10Ehsan, N. and F. Zahra, Castleman disease. 2022.
Laboratory Studies:
The examiner will check for abnormal blood cell counts and other signs of CD. They may also perform HIV tests. Laboratory findings are quite variable across the subtypes of CD. The laboratory tests to diagnose CD are complete blood count, liver function tests, and serum protein electrophoresis. Typical laboratory findings include:
- Anemia
- Thrombocytopenia or Thrombocytosis
- Hypoalbuminemia
- Elevated alkaline phosphatase
- Polyclonal hypergammaglobulinemia
- Elevated C-reactive protein (CRP)
- Elevated fibrinogen
- Elevated erythrocyte sedimentation rate
- Elevated blood urea nitrogen and creatinine
- Elevated IL-6 and lactate dehydrogenase (LDH)
- Elevated vascular endothelial growth factor
- Elevated autoantibodies such as antinuclear antibody
- Positive HIV and HHV-811Talat, N., A.P. Belgaumkar, and K.-M. Schulte, Surgery in Castleman’s disease: a systematic review of 404 published cases. Annals of Surgery, 2012. 255(4): p. 677-684.
Imaging Tests:
Imaging helps determine whether the patient has UCD or MCD. Computed tomography (CT) scan shows a single, enlarged lymph node. Whole-body imaging is considered if the patient is suspected of UCD and MCD. Positron emission tomography (PET) is also considered to determine whether the disease is limited to a single site or other nodes. Hilar lymph nodes and multiple enlarged mediastinal are often observed in HHV-8-associated MCD.
A chest radiograph will show one or more of the following in case of an HHV-8 positive and negative MCD:
- Bilateral pleural effusions
- Mediastinal widening
- Ground glass or bilateral reticular opacities
Lung parenchymal findings on CT scan include:
- Interlobular septal thickening
- Subpleural nodules
- Perobronchovascular thickening
- Patchy rounded areas of consolidation
- Ground glass opacities
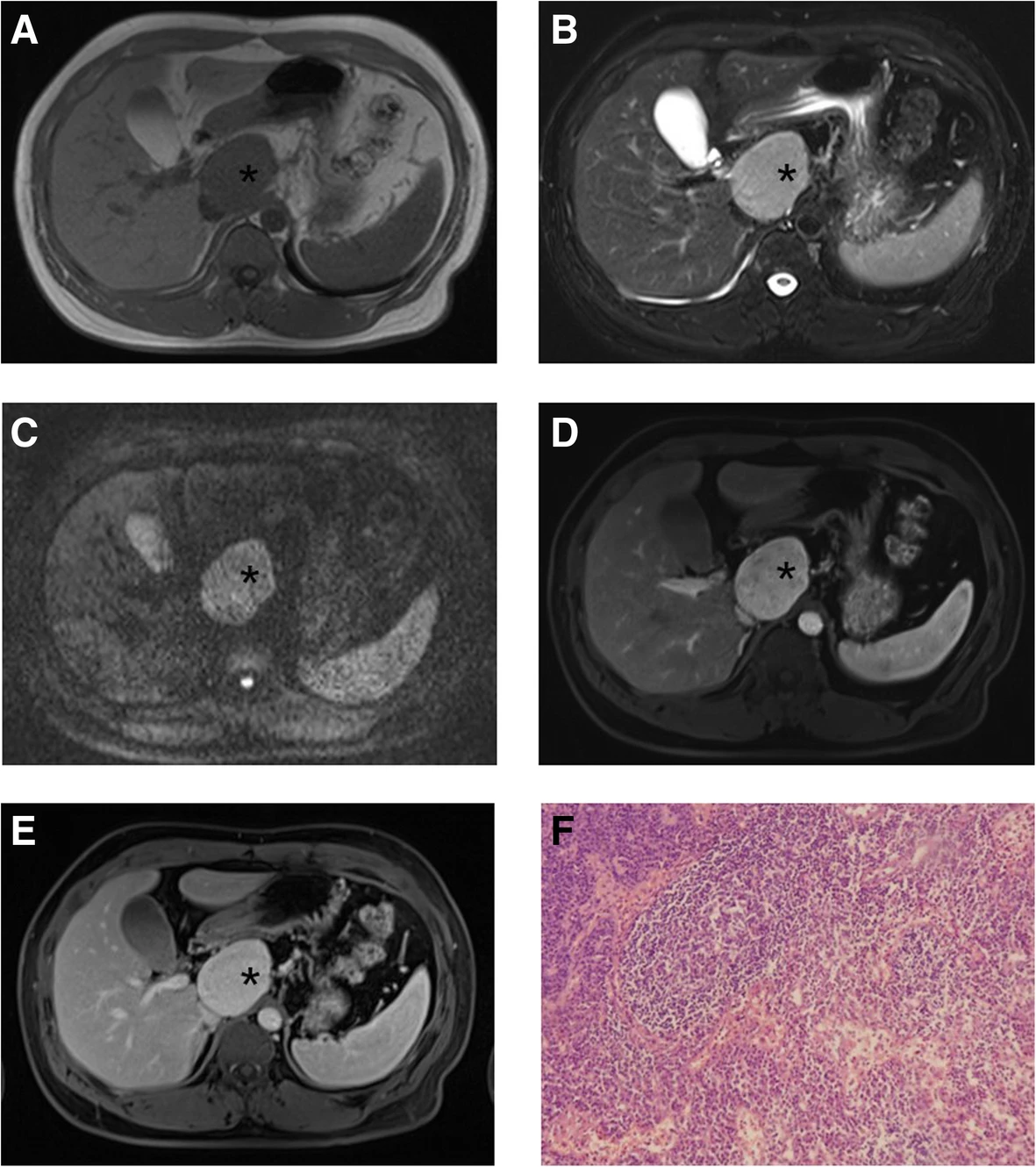
Lymph Node Biopsy:
Excisional lymph node biopsy is the most preferred method of biopsy to establish the diagnosis. Your provider will examine the excised lymph node tissue beneath the microscope to check for signs of disease.
Treatment & Management
CD is a rare disease with a minimum availability of therapeutic approaches.
- In the case of UCD, complete surgical resection of the tumor is the best treatment option. You may need immunotherapy or radiation therapy before the surgery as they can shrink the growth of the tumor on your lymph nodes, making them easier to remove.
- If symptoms are due to compression and inflammatory syndrome, then rituximab and IL-6 therapy are recommended, respectively.
MCD is more challenging to treat than UCD.
- In HHV-8-associated MCD, the highly effective option is chemotherapy with rituximab. For patients with associated HIV infection, antiretroviral therapy is considered.12Bower, M., How I treat HIV-associated multicentric Castleman disease. Blood, The Journal of the American Society of Hematology, 2010. 116(22): p. 4415-4421.
- Doctors also recommend the combination of rituximab with liposomal doxorubicin and prednisone. In the case of HIV-negative patients, doctors consider antiviral therapy with ganciclovir. The IL-6 inhibitor siltuximab is also a preferred therapy. If siltuximab is unavailable, IL-6 inhibitor tocilizumab can be used as a replacement.
Prognosis of Castleman’s Disease
If UCD is eliminated with surgical resection, then it can have a good prognosis. Since MCD involves multiple systemic disorders due to deficient treatment options and non-specific clinical features that often go unnoticed and lead to delayed diagnosis so its prognosis can be awful. Also, MCD and UCD can sometimes progress to non-Hodgkin lymphoma.13Van Rhee, F., A. Greenway, and K. Stone, Treatment of idiopathic Castleman disease. Hematology/Oncology Clinics, 2018. 32(1): p. 89-106.
Final Remarks
The CD is a rare lymphoproliferative disease. Its clinical presentation, laboratory findings, as well as radiological images do not offer much specific information. It can be challenging to distinguish it from other lymphadenopathies. Immediately, go for a checkup as soon as you notice swelling in your lymph node that persists for a longer time. Patients with MCD must go for regular checkups to avoid ant fatal complications.
Refrences
- 1Ehsan, N. and F. Zahra, Castleman disease. 2022.
- 2El Hussein, S., et al., Unicentric Castleman disease: illustration of its morphologic Spectrum and review of the differential diagnosis. Archives of Pathology & Laboratory Medicine, 2024. 148(1): p. 99-106.
- 3Talat, N., A.P. Belgaumkar, and K.-M. Schulte, Surgery in Castleman’s disease: a systematic review of 404 published cases. Annals of Surgery, 2012. 255(4): p. 677-684.
- 4Yu, L., et al., Clinical and pathological characteristics of HIV- and HHV-8–and HHV-8-negative Castleman disease. Blood, The Journal of the American Society of Hematology, 2017. 129(12): p. 1658-1668.
- 5Lang, E. and F. van Rhee, Idiopathic multicentric Castleman disease: an update in diagnosis and treatment advances. Blood Reviews, 2024. 64: p. 101161.
- 6Han, X. and D.-B. Zhou, Advances in etiology and management of Castleman’s disease. Zhongguo yi xue ke xue Yuan xue bao. Acta Academiae Medicinae Sinicae, 2009. 31(5): p. 639-643.
- 7Ehsan, N. and F. Zahra, Castleman disease. 2022.
- 8Van Rhee, F., A. Greenway, and K. Stone, Treatment of idiopathic Castleman disease. Hematology/Oncology Clinics, 2018. 32(1): p. 89-106.
- 9Wang, S., et al., Clinicopathological characteristics of unicentric retroperitoneal Castleman’s disease: a study of 14 cases. World Journal of Surgical Oncology, 2015. 14: p. 1-4.
- 10Ehsan, N. and F. Zahra, Castleman disease. 2022.
- 11Talat, N., A.P. Belgaumkar, and K.-M. Schulte, Surgery in Castleman’s disease: a systematic review of 404 published cases. Annals of Surgery, 2012. 255(4): p. 677-684.
- 12Bower, M., How I treat HIV-associated multicentric Castleman disease. Blood, The Journal of the American Society of Hematology, 2010. 116(22): p. 4415-4421.
- 13Van Rhee, F., A. Greenway, and K. Stone, Treatment of idiopathic Castleman disease. Hematology/Oncology Clinics, 2018. 32(1): p. 89-106.

